当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Redox-couple investigations in Si-doped Li-rich cathode materials
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2021-1-8 , DOI: 10.1039/d0cp05737a Leah Nation 1, 2, 3, 4 , Yan Wu 4, 5, 6 , Xiaoming Liu 4, 7, 8, 9 , Miaofang Chi 4, 7, 8, 9 , Yuqin Wu 4, 10, 11, 12 , Yue Qi 4, 10, 11, 12 , Brian W. Sheldon 1, 2, 3, 4
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2021-1-8 , DOI: 10.1039/d0cp05737a Leah Nation 1, 2, 3, 4 , Yan Wu 4, 5, 6 , Xiaoming Liu 4, 7, 8, 9 , Miaofang Chi 4, 7, 8, 9 , Yuqin Wu 4, 10, 11, 12 , Yue Qi 4, 10, 11, 12 , Brian W. Sheldon 1, 2, 3, 4
Affiliation
|
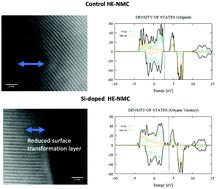
In this investigation, the improved electrochemical behavior in Si-doped Li-rich cathodes is studied with scanning transmission electron microscopy (STEM) and electron energy loss spectroscopy (EELS). Z-contrast images show a layered structure that develops a thin, spinel-like surface layer after the first charge cycle. Si-doping increases discharge capacity by ∼25% and appears to retard the surface phase transformation. Based on electron energy loss spectra, the surface layer in the doped material has an altered oxygen electronic environment, which supports the STEM findings. Furthermore, Si-doping changes the redox behavior during the activation cycle. Density functional theory calculations indicate that Si-doping can increase oxygen vacancy formation, and change the sequence of the redox couples by introducing more oxygen vacancies before or during the typical high voltage activation process. The results of this work indicate that the type of doping employed here is an effective strategy for controlling the complex charge compensation mechanisms in lithium-rich cathodes.
中文翻译:

掺杂硅的富锂正极材料中的氧化还原耦合研究
在这项研究中,使用扫描透射电子显微镜(STEM)和电子能量损失谱(EELS)研究了掺Si富锂阴极中电化学行为的改善。Z对比图像显示了一个分层结构,该结构在第一个充电周期后会形成一个尖晶石状的薄表面层。Si掺杂使放电容量增加约25%,并且似乎阻碍了表面相变。基于电子能量损失谱,掺杂材料中的表面层具有改变的氧电子环境,这支持了STEM的发现。此外,Si掺杂改变了激活周期中的氧化还原行为。密度泛函理论计算表明,硅掺杂可以增加氧空位的形成,在典型的高压活化过程之前或期间,通过引入更多的氧空位来改变氧化还原对的顺序。这项工作的结果表明,此处采用的掺杂类型是控制富锂阴极中复杂电荷补偿机制的有效策略。
更新日期:2021-01-25
中文翻译:

掺杂硅的富锂正极材料中的氧化还原耦合研究
在这项研究中,使用扫描透射电子显微镜(STEM)和电子能量损失谱(EELS)研究了掺Si富锂阴极中电化学行为的改善。Z对比图像显示了一个分层结构,该结构在第一个充电周期后会形成一个尖晶石状的薄表面层。Si掺杂使放电容量增加约25%,并且似乎阻碍了表面相变。基于电子能量损失谱,掺杂材料中的表面层具有改变的氧电子环境,这支持了STEM的发现。此外,Si掺杂改变了激活周期中的氧化还原行为。密度泛函理论计算表明,硅掺杂可以增加氧空位的形成,在典型的高压活化过程之前或期间,通过引入更多的氧空位来改变氧化还原对的顺序。这项工作的结果表明,此处采用的掺杂类型是控制富锂阴极中复杂电荷补偿机制的有效策略。



























 京公网安备 11010802027423号
京公网安备 11010802027423号