当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hydrogen abstraction/addition reactions in soot surface growth
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2020-12-29 , DOI: 10.1039/d0cp06406h Qingzhao Chu 1, 2, 3, 4, 5 , Baolu Shi 2, 3, 4, 5 , Hongyu Wang 1, 2, 3, 4 , Dongping Chen 1, 2, 3, 4 , Lijuan Liao 3, 4, 6, 7, 8
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2020-12-29 , DOI: 10.1039/d0cp06406h Qingzhao Chu 1, 2, 3, 4, 5 , Baolu Shi 2, 3, 4, 5 , Hongyu Wang 1, 2, 3, 4 , Dongping Chen 1, 2, 3, 4 , Lijuan Liao 3, 4, 6, 7, 8
Affiliation
|
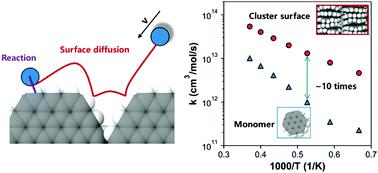
The hydrogen abstraction (HB) and addition reactions (HD) by H radicals are examined on a series of polycyclic aromatic hydrocarbon (PAH) monomers and models of quasi-surfaces using quasi-classical trajectory (QCT) method. QCT results reproduce the rate constants of HB reactions on PAH monomers from density functional theory (DFT) in the range of 1500–2700 K. The PAH size has a minor impact on the rates of HB reactions, especially at temperatures beyond 2100 K. In contrast, HD reactions have a clear size dependence, and a larger PAH yields a higher rate. It was also found that the preferred reaction pathway changes from HB to HD reactions at ∼1900 K. The rates of surface HB and HD reactions exceed those in the gas phase by nearly one factor of magnitude. Further analysis of the detailed trajectory of the QCT method reveals that about 50% of surface reactions can be attributed to the events of surface diffusion, which depends on the local energy transfer in gas-surface interactions. However, this phenomenon is not preferred in PAH monomers, as expected. Our finding here questions the treatment of the surface reactions of soot as the product of the first collision between the gaseous species and particle surface. The surface diffusion-induced reactions should be accounted for in the rates of the surface HB and HD reactions. The rate constants of HB and HD reactions on each reactive site (surface zig-zag, surface free-edge and pocket free-edge sites) were calculated by QCT method, and are recommended for the further development of surface chemistry models in soot formation.
中文翻译:

烟灰表面生长中的氢提取/加成反应
使用准经典轨迹(QCT)方法在一系列多环芳烃(PAH)单体和准表面模型上检查了H自由基的氢提取(HB)和加成反应(HD)。QCT结果根据密度泛函理论(DFT)再现了HB反应在PAH单体上的速率常数,范围为1500–2700K。PAH尺寸对HB反应的速率影响较小,尤其是在2100 K以上的温度下。相反,HD反应具有明显的尺寸依赖性,而较大的PAH产生较高的速率。还发现,在约1900 K时,优选的反应途径从HB反应转变为HD反应。表面HB和HD反应的速率比气相反应速率快将近一个数量级。对QCT方法的详细轨迹的进一步分析表明,约50%的表面反应可归因于表面扩散事件,这取决于气-气相互作用中的局部能量转移。但是,正如预期的那样,这种现象在PAH单体中不是优选的。我们在这里的发现对作为气态物质与颗粒表面之间第一次碰撞产物的烟尘表面反应的处理提出了质疑。表面扩散引起的反应应在表面HB和HD反应的速率中加以考虑。通过QCT方法计算每个反应位点(表面之字形,表面自由边缘和袋状自由边缘位点)的HB和HD反应速率常数,建议将其用于进一步发展烟灰形成中的表面化学模型。
更新日期:2021-01-25
中文翻译:

烟灰表面生长中的氢提取/加成反应
使用准经典轨迹(QCT)方法在一系列多环芳烃(PAH)单体和准表面模型上检查了H自由基的氢提取(HB)和加成反应(HD)。QCT结果根据密度泛函理论(DFT)再现了HB反应在PAH单体上的速率常数,范围为1500–2700K。PAH尺寸对HB反应的速率影响较小,尤其是在2100 K以上的温度下。相反,HD反应具有明显的尺寸依赖性,而较大的PAH产生较高的速率。还发现,在约1900 K时,优选的反应途径从HB反应转变为HD反应。表面HB和HD反应的速率比气相反应速率快将近一个数量级。对QCT方法的详细轨迹的进一步分析表明,约50%的表面反应可归因于表面扩散事件,这取决于气-气相互作用中的局部能量转移。但是,正如预期的那样,这种现象在PAH单体中不是优选的。我们在这里的发现对作为气态物质与颗粒表面之间第一次碰撞产物的烟尘表面反应的处理提出了质疑。表面扩散引起的反应应在表面HB和HD反应的速率中加以考虑。通过QCT方法计算每个反应位点(表面之字形,表面自由边缘和袋状自由边缘位点)的HB和HD反应速率常数,建议将其用于进一步发展烟灰形成中的表面化学模型。











































 京公网安备 11010802027423号
京公网安备 11010802027423号