Journal of Environmental Chemical Engineering ( IF 7.4 ) Pub Date : 2021-01-23 , DOI: 10.1016/j.jece.2021.105119 Jaegwan Shin , Yong-Gu Lee , Jinwoo Kwak , Sangwon Kim , Sang-Ho Lee , Yongeun Park , Sang-Deok Lee , Kangmin Chon
|
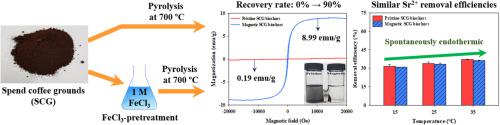
This study rigorously investigated the adsorption behaviors and mechanisms of strontium ions (Sr2+) by the pristine and magnetic biochars prepared via the pyrolysis of pristine and FeCl3-pretreated spent coffee grounds (SCG). The FeCl3-pretreatment could magnetize successfully the pristine SCG biochars (magnetization value of pristine SCG biochars = 0.19 emu/g; magnetization value of magnetic SCG biochars = 8.99 emu/g) and improved substantially their specific surface area (pristine SCG biochars = 5.3 m2/g; magnetic SCG biochars = 431.7 m2/g) and total pore volume (pristine SCG biochars = 0.002 cm3/g; magnetic SCG biochars = 0.186 cm3/g). However, the removal efficiency of Sr2+ with the pristine SCG biochars (33.4–34.1%) was not significantly different from that with the magnetic SCG biochars (33.1–33.9%). The calculated kinetic parameters indicate that the adsorption of Sr2+ by the pristine and magnetic SCG biochars was governed by the chemisorption (R2 of the pseudo-first-order kinetic equation ≤ 0.602; R2 of the pseudo-second-order kinetic equation = 1.000). The higher R2 values of the Langmuir isotherm (R2 = 0.999) model compared to the Freundlich isotherm model (R2 ≤ 0.950) are evident that the monolayer adsorption mainly determined the adsorptive removal of Sr2+ with the pristine and magnetic SCG biochars. Moreover, the adsorption of Sr2+ using the pristine and magnetic SCG biochars was the spontaneous and endothermic process. These observations indicate that the FeCl3-pretreatment might offer a viable way to effectively recover the residual SCG biochars after the Sr2+ adsorption through the magnetic separation (recovery rate = 90%).
中文翻译:

来自废咖啡渣的原始和磁性生物炭对放射性锶的吸附
这项研究严格研究了通过原始和FeCl 3预处理的废咖啡渣(SCG)的热解制备的原始和磁性生物炭对锶离子(Sr 2+)的吸附行为和机理。FeCl 3预处理可以成功地磁化原始SCG生物炭(原始SCG生物炭的磁化值= 0.19 emu / g;磁性SCG生物炭的磁化值= 8.99 emu / g)并显着提高其比表面积(原始SCG生物炭= 5.3)。 m 2 / g;磁性SCG生物炭= 431.7 m 2 / g)和总孔体积(原始SCG生物炭= 0.002 cm 3 / g;磁性SCG生物炭= 0.186 cm 3/G)。但是,原始SCG生物炭对Sr 2+的去除效率(33.4–34.1%)与磁性SCG生物炭对Sr 2+的去除效率(33.1–33.9%)没有显着差异。所计算的动力学参数表明,Sr的吸附2+由原始和磁性SCG生物炭由化学吸附(R支配2伪一级动力学方程≤0.602; R 2伪二级动力学方程的= 1.000)。越高- [R 2个Langmuir等温(R值2 = 0.999)模型相比,Freundlich等温模型(R 2 ≤0.950)是明显的是,单分子层吸附主要是确定的吸附除去锶2+与原始和磁性SCG生物炭。此外,使用原始和磁性SCG生物炭吸附Sr 2+是自发的和吸热的过程。这些观察结果表明,FeCl 3预处理可能为通过磁分离吸附Sr 2+后有效回收残留SCG生物炭提供了可行的方法(回收率= 90%)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号