Journal of Environmental Chemical Engineering ( IF 7.4 ) Pub Date : 2021-01-23 , DOI: 10.1016/j.jece.2021.105124 Veer Singh , Jyoti Singh , Vishal Mishra
|
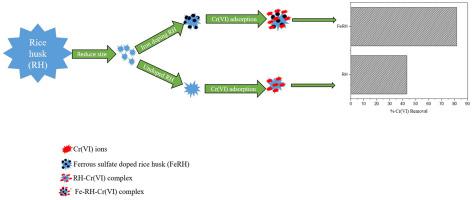
Iron ions were doped on the rice husk for adsorption-cum-reduction of lethal Cr (VI) to less toxic and partially soluble Cr (III). Energy dispersive X-ray analysis and X-ray photoelectric spectroscopy disclosed the effective doping of iron on rice husk and significant reduction of Cr (VI) into Cr (III). Physico-chemical analysis exhibited that the surface of an iron doped rice husk was rough and in houses amino and hydroxyl moieties in majority together with high organic content. The maximum removal of Cr (VI) ions was observed in acidic range as the value of point zero charge was 3.32. The study of dimensionless numbers (φ, Nk and λ) disclosed that the Cr (VI) adsorption on iron coated rice husk was principally controlled by diffusion. The suitability of acidic pH indicated that heavy protonation at FeRH surface generated the electrostatic pull of negatively charged Cr (VI) species, which led to a favorable biosorption. Only 1.32% and 0.18% deviation in experimental results was observed for undoped and iron doped rice husk, respectively in artificial neural network’s correlation plot. The film and pore diffusivity coefficients along with Bangham’s model study revealed that the adsorption was film diffusion limited for both types of rice husks. The maximal Cr (VI) removal was found to be 81.56% for iron doped and 43.28% for undoped rice husk. The iron doped rice husk had high regeneration capacity up to three cycles of adsorption and desorption. Cr (VI) adsorption was chemisorptive, spontaneous, endothermic, favorable and multilayer in nature.
中文翻译:

开发一种经济高效,可循环使用和可行的掺杂金属离子的吸附剂,用于同时吸附和还原有毒的Cr(VI)离子
将铁离子掺入稻壳中,以将致死的六价铬(VI)吸附和还原为毒性较小的部分可溶性六价铬(III)。能量色散X射线分析和X射线光电光谱揭示了铁在稻壳上的有效掺杂以及将Cr(VI)显着还原为Cr(III)。物理化学分析表明,铁掺杂的稻壳表面粗糙,并且内部大部分为氨基和羟基,同时有机物含量较高。零酸电荷值为3.32,可在酸性范围内最大程度地除去Cr(VI)离子。无量纲数(φ,N k和λ)公开了在铁包覆的稻壳上的Cr(VI)吸附主要受扩散控制。酸性pH的适用性表明FeRH表面的重质子化产生带负电的Cr(VI)物质的静电拉力,从而导致良好的生物吸附。在人工神经网络的相关图中,未掺杂和铁掺杂的稻壳的实验结果分别只有1.32%和0.18%的偏差。膜和孔的扩散系数以及Bangham模型研究表明,两种稻壳的吸附都受到膜扩散的限制。发现铁掺杂的最大Cr(VI)去除率为81.56%,未掺杂稻壳的最大Cr(VI)去除为43.28%。铁掺杂的稻壳具有高达三个吸附和解吸循环的高再生能力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号