Tetrahedron Letters ( IF 1.8 ) Pub Date : 2021-01-23 , DOI: 10.1016/j.tetlet.2021.152826 Anastasia A. Fesenko , Anatoly D. Shutalev
|
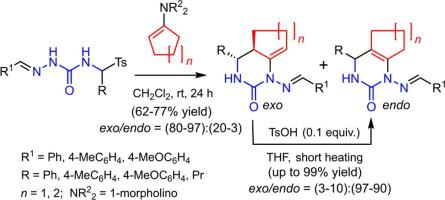
An efficient synthesis of 1-arylideneamino-substituted hexahydro-1H-cyclopenta[d]pyrimidin-2-ones and octahydroquinazolin-2-ones has been developed. The synthesis involves a stereo- and regioselective cascade reaction of the corresponding 4-(tosylmethyl)semicarbazones with 1-morpholinocyclopentene or 1-morpholinocyclohexene to give predominantly bicyclic pyrimidines with an exocyclic C C double bond (80–97%). The later undergo rapid isomerization when heated in THF in the presence of TsOH to form bicyclic pyrimidines with a significant predominance of those with an endocyclic C
C double bond (80–97%). The later undergo rapid isomerization when heated in THF in the presence of TsOH to form bicyclic pyrimidines with a significant predominance of those with an endocyclic C C double bond (90–97%).
C double bond (90–97%).
中文翻译:

烯胺与半氨基甲酰胺基烷基化试剂的反应:环化的1-氨基嘧啶-2-酮衍生物的直接合成
已经开发出有效合成1-芳基氨基取代的六氢-1 H-环戊[ d ]嘧啶-2-酮和八氢喹唑啉-2-酮的方法。合成涉及相应的4-(甲苯磺酰基甲基)半咔唑酮与1-吗啉代环戊烯或1-吗啉代环己烯的立体和区域选择性级联反应,主要产生带有环外C  C双键的双环嘧啶(80-97%)。当在TsOH存在下在THF中加热时,后者会经历快速异构化反应,从而形成双环嘧啶,其显着优势是具有内环C
C双键的双环嘧啶(80-97%)。当在TsOH存在下在THF中加热时,后者会经历快速异构化反应,从而形成双环嘧啶,其显着优势是具有内环C  C双键的双环嘧啶(90-97%)。
C双键的双环嘧啶(90-97%)。



























 京公网安备 11010802027423号
京公网安备 11010802027423号