Chemosphere ( IF 8.1 ) Pub Date : 2021-01-23 , DOI: 10.1016/j.chemosphere.2021.129731 Haeju Kim , Dawoon Jang , Seungjoo Choi , Jeongho Kim , Sungjin Park
|
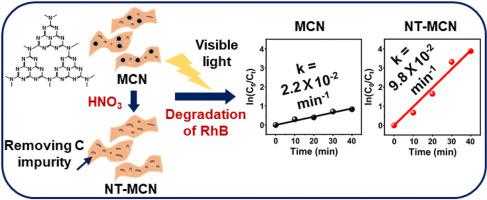
Three-dimensional (3D) carbon nitride (C3N4) can be used as a metal-free platform for visible-light-active photocatalysts because of its suitable band positions. This study reports that HNO3 activation improves the photocatalytic activity of 3D melamine-derived C3N4 (MCN) materials, which degrade the organic pollutant rhodamine B (RhB). HNO3 treatment under reflux removes the carbonaceous impurities in MCN and introduces oxygen-containing functional groups on its surface. Under visible light irradiation, the nitric acid treated MCN (NT-MCN) completely degrades RhB within 30 min. Photophysical characterizations and control experiments with radical scavengers reveal that MCN and NT-MCN follow different reaction mechanisms. Because NT-MCN exhibits a longer photoluminescence lifetime, smaller electrochemical resistance, and larger photocurrent than those of MCN, it enables a more efficient and facile transfer of photogenerated charge carriers for the degradation reaction.
中文翻译:

酸活化的碳氮化物作为光催化剂,在可见光下降解有机污染物
三维(3D)氮化碳(C 3 N 4)由于其合适的能带位置,可以用作可见光活性光催化剂的无金属平台。这项研究报告说,HNO 3活化可改善3D三聚氰胺衍生的C 3 N 4(MCN)材料的光催化活性,从而降解有机污染物若丹明B(RhB)。HNO 3回流下的处理除去了MCN中的碳质杂质,并在其表面引入了含氧官能团。在可见光照射下,硝酸处理的MCN(NT-MCN)在30分钟内完全降解RhB。自由基清除剂的光物理特征和控制实验表明,MCN和NT-MCN遵循不同的反应机理。由于NT-MCN与MCN相比,具有更长的光致发光寿命,更小的电化学电阻和更大的光电流,因此它能够更有效,更轻松地转移光生电荷载流子,以进行降解反应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号