Bioorganic & Medicinal Chemistry ( IF 3.5 ) Pub Date : 2021-01-24 , DOI: 10.1016/j.bmc.2020.115990 Leah N Makley 1 , Oleta T Johnson 2 , Phani Ghanakota 3 , Jennifer N Rauch 2 , Delaney Osborn 2 , Taia S Wu 2 , Tomasz Cierpicki 4 , Heather A Carlson 3 , Jason E Gestwicki 2
|
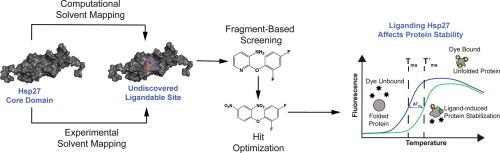
Destabilizing mutations in small heat shock proteins (sHsps) are linked to multiple diseases; however, sHsps are conformationally dynamic, lack enzymatic function and have no endogenous chemical ligands. These factors render sHsps as classically “undruggable” targets and make it particularly challenging to identify molecules that might bind and stabilize them. To explore potential solutions, we designed a multi-pronged screening workflow involving a combination of computational and biophysical ligand-discovery platforms. Using the core domain of the sHsp family member Hsp27/HSPB1 (Hsp27c) as a target, we applied mixed solvent molecular dynamics (MixMD) to predict three possible binding sites, which we confirmed using NMR-based solvent mapping. Using this knowledge, we then used NMR spectroscopy to carry out a fragment-based drug discovery (FBDD) screen, ultimately identifying two fragments that bind to one of these sites. A medicinal chemistry effort improved the affinity of one fragment by ~50-fold (16 µM), while maintaining good ligand efficiency (~0.32 kcal/mol/non-hydrogen atom). Finally, we found that binding to this site partially restored the stability of disease-associated Hsp27 variants, in a redox-dependent manner. Together, these experiments suggest a new and unexpected binding site on Hsp27, which might be exploited to build chemical probes.
中文翻译:

使用计算机溶剂图谱和生物物理方法对 Hsp27/HSPB1 上的可成药位点进行化学验证
小热休克蛋白 (sHsps) 的不稳定突变与多种疾病有关;然而,sHsps 具有构象动态,缺乏酶功能并且没有内源化学配体。这些因素使得小热激蛋白成为典型的“不可成药”靶标,并且使得识别可能结合和稳定它们的分子变得特别具有挑战性。为了探索潜在的解决方案,我们设计了一个多管齐下的筛选工作流程,涉及计算和生物物理配体发现平台的组合。使用 sHsp 家族成员 Hsp27/HSPB1 (Hsp27c) 的核心结构域作为靶标,我们应用混合溶剂分子动力学 (MixMD) 来预测三个可能的结合位点,并使用基于 NMR 的溶剂图谱证实了这一点。利用这一知识,我们随后使用核磁共振波谱进行基于片段的药物发现 (FBDD) 筛选,最终识别出与这些位点之一结合的两个片段。一项药物化学工作将一个片段的亲和力提高了约 50 倍 (16 µM),同时保持良好的配体效率(约 0.32 kcal/mol/非氢原子)。最后,我们发现与该位点的结合以氧化还原依赖性方式部分恢复了疾病相关 Hsp27 变体的稳定性。总之,这些实验表明 Hsp27 上存在一个新的、意想不到的结合位点,可以利用它来构建化学探针。



























 京公网安备 11010802027423号
京公网安备 11010802027423号