Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iodonium salts as efficient iodine(III)-based noncovalent organocatalysts for Knorr-type reactions
RSC Advances ( IF 3.9 ) Pub Date : 2021-1-22 , DOI: 10.1039/d0ra09640g Sevilya N Yunusova 1 , Alexander S Novikov 1 , Natalia S Soldatova 1 , Mikhail A Vovk 2 , Dmitrii S Bolotin 1
RSC Advances ( IF 3.9 ) Pub Date : 2021-1-22 , DOI: 10.1039/d0ra09640g Sevilya N Yunusova 1 , Alexander S Novikov 1 , Natalia S Soldatova 1 , Mikhail A Vovk 2 , Dmitrii S Bolotin 1
Affiliation
|
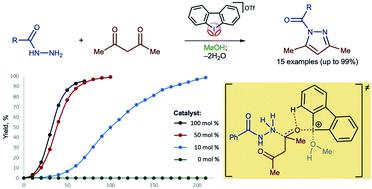
Hypervalent iodine(III)-derivatives display higher catalytic activity than other aliphatic and aromatic iodine(I)– or bromine(I)-containing substrates for a Knorr-type reaction of N-acetyl hydrazides with acetyl acetone to give N-acyl pyrazoles. The highest activity was observed for dibenziodolium triflate, for which 10 mol% resulted in the generation of N-acyl pyrazole from acyl hydrazide and acetyl acetone typically at 50 °C for 3.5–6 h with up to 99% isolated yields. 1H NMR titration data and DFT calculations indicate that the catalytic activity of the iodine(III) is caused by the binding with a ketone.
中文翻译:

碘鎓盐作为克诺尔型反应的有效碘(III)基非共价有机催化剂
对于N-乙酰酰肼与乙酰丙酮的克诺尔型反应生成N-酰基吡唑,高价碘 ( III ) 衍生物比其他脂肪族和芳香族含碘 () 或溴 () 的底物表现出更高的催化活性。三氟甲磺酸二苯并碘鎓的活性最高,10 mol% 会导致酰肼和乙酰丙酮在 50 °C 下反应 3.5-6 小时,生成N-酰基吡唑,分离收率高达 99%。 1 H NMR滴定数据和DFT计算表明碘( III )的催化活性是由与酮的结合引起的。
更新日期:2021-01-22
中文翻译:

碘鎓盐作为克诺尔型反应的有效碘(III)基非共价有机催化剂
对于N-乙酰酰肼与乙酰丙酮的克诺尔型反应生成N-酰基吡唑,高价碘 ( III ) 衍生物比其他脂肪族和芳香族含碘 () 或溴 () 的底物表现出更高的催化活性。三氟甲磺酸二苯并碘鎓的活性最高,10 mol% 会导致酰肼和乙酰丙酮在 50 °C 下反应 3.5-6 小时,生成N-酰基吡唑,分离收率高达 99%。 1 H NMR滴定数据和DFT计算表明碘( III )的催化活性是由与酮的结合引起的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号