当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The H2+ + HD reaction at low collision energies: H3+/H2D+ branching ratio and product-kinetic-energy distributions
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2021-1-22 , DOI: 10.1039/d0cp06107g Katharina Höveler 1, 2, 3, 4 , Johannes Deiglmayr 1, 2, 3, 4 , Josef A. Agner 1, 2, 3, 4 , Hansjürg Schmutz 1, 2, 3, 4 , Frédéric Merkt 1, 2, 3, 4
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2021-1-22 , DOI: 10.1039/d0cp06107g Katharina Höveler 1, 2, 3, 4 , Johannes Deiglmayr 1, 2, 3, 4 , Josef A. Agner 1, 2, 3, 4 , Hansjürg Schmutz 1, 2, 3, 4 , Frédéric Merkt 1, 2, 3, 4
Affiliation
|
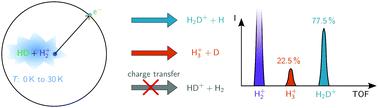
The fully state-selected reactions between H2+ molecules in the X+ 2Σg+(v+ = 0, N+ = 0) state and HD molecules in the X 1Σg+(v = 0, J = 0) state forming H3+ + D and H2D+ + H have been studied at collision energies Ecoll between 0 and kB·30 K with a resolution of about 75 mK at the lowest energies. H2 molecules in a supersonic beam were prepared in Rydberg-Stark states with principal quantum number n = 27 and merged with a supersonic beam of ground-state HD molecules using a curved surface-electrode Rydberg-Stark decelerator and deflector. The reaction between H2+ and HD was studied within the orbit of the Rydberg electron to avoid heating of the ions by stray electric fields. The reaction was observed for well-defined and adjustable time intervals, called reaction-observation windows, between two electric-field pulses. The first pulse swept all ions away from the reaction volume and its falling edge defined the beginning of the reaction-observation window. The second pulse extracted the product ions toward a charged-particle detector located at the end of a time-of-flight tube and its rising edge defined the end of the reaction-observation window. Monitoring and analysing the time-of-flight distributions of the H3+ and H2D+ products in dependence of the duration of the reaction-observation window enabled us to obtain information on the kinetic-energy distribution of the product ions and determine branching ratios of the H3+ + D and H2D+ + H reaction channels. The mean product-kinetic-energy release is 0.46(5) eV, representing 27(3)% of the available energy, and the H3+ + D product branching ratio is 0.225(20). The relative reaction rates correspond closely to Langevin capture rates down to the lowest energies probed experimentally (≈kB·50 mK).
中文翻译:

低碰撞能量下的H2 + HD反应:H3 + / H2D +支化比和产物动力学能量分布
h的完全状态选择的反应2个+在X分子+ 2 Σ克+(v + = 0,Ñ + = 0)在X状态和HD分子1 Σ克+(v = 0,Ĵ = 0)研究了在碰撞能量E coll在0至k B ·30 K之间时形成H 3 + + D和H 2 D + + H的状态,在最低能量下分辨率约为75 mK。高2超声束中的分子以主量子数n = 27的Rydberg-Stark状态制备,并使用曲面电极Rydberg-Stark减速器和偏转器与基态HD分子的超声束合并。H 2 +之间的反应为了避免杂散电场加热离子,对HD进行了Rydberg电子轨道研究。在两个电场脉冲之间以明确定义且可调的时间间隔(称为反应观察窗口)观察反应。第一个脉冲将所有离子从反应体积中清除,其下降沿定义了反应观察窗口的起点。第二个脉冲将产物离子引向位于飞行时间管末端的带电粒子检测器,其上升沿定义了反应观察窗口的末端。监视和分析H 3 +和H 2 D +的飞行时间分布取决于反应观察窗口持续时间的产物,使我们能够获得有关产物离子的动能分布的信息,并确定H 3 + + D和H 2 D + + H反应通道的分支比。产物的平均动能释放为0.46(5)eV,占可用能量的27(3)%,H 3 + + D产物的分支比为0.225(20)。相对反应速率密切地对应于郎之万捕获率下降到最低能量探测实验(≈ ķ乙·50 MK)。
更新日期:2021-01-22
中文翻译:

低碰撞能量下的H2 + HD反应:H3 + / H2D +支化比和产物动力学能量分布
h的完全状态选择的反应2个+在X分子+ 2 Σ克+(v + = 0,Ñ + = 0)在X状态和HD分子1 Σ克+(v = 0,Ĵ = 0)研究了在碰撞能量E coll在0至k B ·30 K之间时形成H 3 + + D和H 2 D + + H的状态,在最低能量下分辨率约为75 mK。高2超声束中的分子以主量子数n = 27的Rydberg-Stark状态制备,并使用曲面电极Rydberg-Stark减速器和偏转器与基态HD分子的超声束合并。H 2 +之间的反应为了避免杂散电场加热离子,对HD进行了Rydberg电子轨道研究。在两个电场脉冲之间以明确定义且可调的时间间隔(称为反应观察窗口)观察反应。第一个脉冲将所有离子从反应体积中清除,其下降沿定义了反应观察窗口的起点。第二个脉冲将产物离子引向位于飞行时间管末端的带电粒子检测器,其上升沿定义了反应观察窗口的末端。监视和分析H 3 +和H 2 D +的飞行时间分布取决于反应观察窗口持续时间的产物,使我们能够获得有关产物离子的动能分布的信息,并确定H 3 + + D和H 2 D + + H反应通道的分支比。产物的平均动能释放为0.46(5)eV,占可用能量的27(3)%,H 3 + + D产物的分支比为0.225(20)。相对反应速率密切地对应于郎之万捕获率下降到最低能量探测实验(≈ ķ乙·50 MK)。



























 京公网安备 11010802027423号
京公网安备 11010802027423号