当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The crystallization enthalpy and entropy of protein solutions: microcalorimetry, van't Hoff determination and linearized Poisson–Boltzmann model of tetragonal lysozyme crystals
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2021-1-15 , DOI: 10.1039/d0cp06113a Lorena Hentschel 1, 2, 3, 4 , Jan Hansen 1, 2, 3, 4 , Stefan U. Egelhaaf 1, 2, 3, 4 , Florian Platten 1, 2, 3, 4, 5
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2021-1-15 , DOI: 10.1039/d0cp06113a Lorena Hentschel 1, 2, 3, 4 , Jan Hansen 1, 2, 3, 4 , Stefan U. Egelhaaf 1, 2, 3, 4 , Florian Platten 1, 2, 3, 4, 5
Affiliation
|
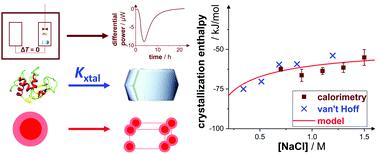
During a first-order phase transition, a thermodynamic system releases or absorbs latent heat. Despite their fundamental importance, the heat or enthalpy change occurring during protein crystallization has been directly measured only in a few cases, and the associated entropy change can only be determined indirectly. This work provides an experimental determination and theoretical analysis of the dependence of the molar crystallization enthalpy of lysozyme solutions, ΔHxtal, on the physicochemical solution parameters. Its value is determined directly by isothermal microcalorimetry and indirectly by a van't Hoff analysis of solubility data, which quantitatively agree. This suggests a two-state crystallization process, in which oligomeric intermediates play a minor role. ΔHxtal is found to be negative on the order of few tens of the thermal energy per molecule. It is independent of protein concentration and stirring speed, but weakly depends on salt (NaCl) concentration and solution pH. Assuming that crystals are electrostatically neutral, these trends are explained by a linearized Poisson–Boltzmann theory. In addition, the molar crystallization entropy, ΔSxtal, is analyzed. The dependence of the van't Hoff entropy on salt concentration and pH is captured by the model, complementing the analysis of crystallization thermodynamics.
中文翻译:

蛋白质溶液的结晶焓和熵:微量量热法,van't Hoff测定和四方溶菌酶晶体的线性泊松-玻耳兹曼模型
在一级相变过程中,热力学系统释放或吸收潜热。尽管它们具有根本的重要性,但仅在少数情况下可以直接测量蛋白质结晶过程中发生的热量或焓变,并且只能间接确定相关的熵变。这项工作提供了溶菌酶溶液的摩尔结晶焓ΔH xtal对理化溶液参数的依赖性的实验确定和理论分析。它的值直接通过等温微量热法测定,间接通过溶解度数据的van't Hoff分析来定量确定。这表明了两态结晶过程,其中低聚中间体起着较小的作用。Δ ^ h发现xtal为负数,约为每个分子数十个热能。它与蛋白质浓度和搅拌速度无关,但在很大程度上取决于盐(NaCl)浓度和溶液pH值。假设晶体是静电中性的,这些趋势可以用线性泊松-玻尔兹曼理论来解释。另外,分析了摩尔结晶熵ΔS xtal。该模型捕获了范霍夫熵对盐浓度和pH的依赖性,补充了结晶热力学分析。
更新日期:2021-01-22
中文翻译:

蛋白质溶液的结晶焓和熵:微量量热法,van't Hoff测定和四方溶菌酶晶体的线性泊松-玻耳兹曼模型
在一级相变过程中,热力学系统释放或吸收潜热。尽管它们具有根本的重要性,但仅在少数情况下可以直接测量蛋白质结晶过程中发生的热量或焓变,并且只能间接确定相关的熵变。这项工作提供了溶菌酶溶液的摩尔结晶焓ΔH xtal对理化溶液参数的依赖性的实验确定和理论分析。它的值直接通过等温微量热法测定,间接通过溶解度数据的van't Hoff分析来定量确定。这表明了两态结晶过程,其中低聚中间体起着较小的作用。Δ ^ h发现xtal为负数,约为每个分子数十个热能。它与蛋白质浓度和搅拌速度无关,但在很大程度上取决于盐(NaCl)浓度和溶液pH值。假设晶体是静电中性的,这些趋势可以用线性泊松-玻尔兹曼理论来解释。另外,分析了摩尔结晶熵ΔS xtal。该模型捕获了范霍夫熵对盐浓度和pH的依赖性,补充了结晶热力学分析。











































 京公网安备 11010802027423号
京公网安备 11010802027423号