Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interfacial sites in platinum−hydroxide−cobalt hybrid nanostructures for promoting CO oxidation activity
Nanoscale ( IF 5.8 ) Pub Date : 2020-12-18 , DOI: 10.1039/d0nr07880h Wenxue He 1, 2, 3, 4 , Li Huang 1, 2, 3, 4 , Chengyong Liu 1, 2, 3, 4 , Siyu Wang 1, 2, 3, 4 , Zhixin Long 1, 2, 3, 4 , Fengchun Hu 1, 2, 3, 4 , Zhihu Sun 1, 2, 3, 4
Nanoscale ( IF 5.8 ) Pub Date : 2020-12-18 , DOI: 10.1039/d0nr07880h Wenxue He 1, 2, 3, 4 , Li Huang 1, 2, 3, 4 , Chengyong Liu 1, 2, 3, 4 , Siyu Wang 1, 2, 3, 4 , Zhixin Long 1, 2, 3, 4 , Fengchun Hu 1, 2, 3, 4 , Zhihu Sun 1, 2, 3, 4
Affiliation
|
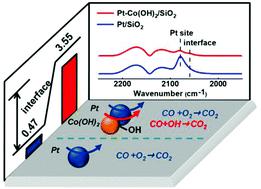
Metal–oxide/hydroxide hybrid nanostructures provide an excellent platform to study the interfacial effects on tailoring the catalysis of metal catalysts. Herein, a hybrid nanostructure of Pt@Co(OH)2 supported on SiO2 was synthesized by incipient wetness impregnation of Co(OH)2 with the aid of H2O2 and successive urea-assisted deposition−precipitation of platinum nanoparticles. The Fenton-like reaction between Co2+ and H2O2 during the impregnation process facilitates the formation of active interfacial sites. This hybrid nanostructure exhibits much higher catalytic activity towards CO oxidation than Pt/SiO2 nanoparticles with a similar Pt loading and particle size. In situ diffuse reflectance infrared Fourier transform spectroscopy was used to track the CO adsorption processes and to identify the reaction intermediates during CO oxidation. It shows that the OH species at the Pt–OH–Co interfacial sites could readily react with CO adsorbed on neighboring Pt to yield CO2 by forming *COOH intermediates and oxygen vacancies. Under the CO + O2 oxidation conditions, O2 molecules are activated by the oxygen vacancy and react with the CO molecules adsorbed on Pt to generate CO2, via forming the highly active *OOH intermediates as observed by DRIFTS.
中文翻译:

铂-氢氧化物-钴杂化纳米结构中的界面部位可促进CO氧化活性
金属-氧化物/氢氧化物杂化纳米结构为研究界面效应对定制金属催化剂的催化作用提供了一个极好的平台。在此,Pt中的@的Co(OH)的混合纳米结构2支撑在SiO 2中的Co(OH)的初始润湿浸渍合成2与H的援助2 Ò 2和铂纳米颗粒的连续尿素辅助沉积-沉淀。浸渍过程中Co 2 +和H 2 O 2之间的Fenton样反应促进了活性界面部位的形成。这种杂化纳米结构显示出比Pt / SiO 2高得多的对CO氧化的催化活性。具有相似的Pt负载和粒径的纳米粒子。原位漫反射红外傅里叶变换光谱用于跟踪CO的吸附过程并确定CO氧化过程中的反应中间体。结果表明,Pt-OH-Co界面处的OH种类很容易与相邻Pt上吸附的CO反应,通过形成* COOH中间体和氧空位而产生CO 2。下的CO + O 2的氧化条件下,O- 2分子被所述氧空位活化并与吸附在铂产生CO的CO分子反应2,通过由作为DRIFTS观察到形成高活性* OOH中间体。
更新日期:2021-01-22
中文翻译:

铂-氢氧化物-钴杂化纳米结构中的界面部位可促进CO氧化活性
金属-氧化物/氢氧化物杂化纳米结构为研究界面效应对定制金属催化剂的催化作用提供了一个极好的平台。在此,Pt中的@的Co(OH)的混合纳米结构2支撑在SiO 2中的Co(OH)的初始润湿浸渍合成2与H的援助2 Ò 2和铂纳米颗粒的连续尿素辅助沉积-沉淀。浸渍过程中Co 2 +和H 2 O 2之间的Fenton样反应促进了活性界面部位的形成。这种杂化纳米结构显示出比Pt / SiO 2高得多的对CO氧化的催化活性。具有相似的Pt负载和粒径的纳米粒子。原位漫反射红外傅里叶变换光谱用于跟踪CO的吸附过程并确定CO氧化过程中的反应中间体。结果表明,Pt-OH-Co界面处的OH种类很容易与相邻Pt上吸附的CO反应,通过形成* COOH中间体和氧空位而产生CO 2。下的CO + O 2的氧化条件下,O- 2分子被所述氧空位活化并与吸附在铂产生CO的CO分子反应2,通过由作为DRIFTS观察到形成高活性* OOH中间体。











































 京公网安备 11010802027423号
京公网安备 11010802027423号