当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iridium-catalyzed enantioselective olefinic C(sp2)–H allylic alkylation
Chemical Science ( IF 7.6 ) Pub Date : 2021-1-15 , DOI: 10.1039/d0sc06208a Rahul Sarkar 1 , Santanu Mukherjee 1
Chemical Science ( IF 7.6 ) Pub Date : 2021-1-15 , DOI: 10.1039/d0sc06208a Rahul Sarkar 1 , Santanu Mukherjee 1
Affiliation
|
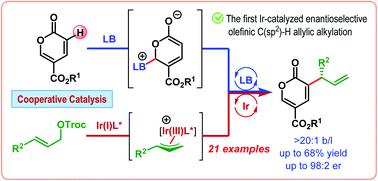
The first iridium-catalyzed enantioselective olefinic C(sp2)–H allylic alkylation is developed in cooperation with Lewis base catalysis. This reaction, catalyzed by cinchonidine and an in situ generated cyclometalated Ir(I)/phosphoramidite complex, makes use of the latent enolate character of an α,β-unsaturated carbonyl compound, namely coumalate ester, to introduce an allyl group at its α-position in a branched-selective manner in moderate to good yield with good to excellent enantioselectivities (up to 98 : 2 er).
中文翻译:

铱催化对映选择性烯属C(sp2)–H烯丙基烷基化
第一个铱催化的对映选择性烯属 C(sp 2 )–H 烯丙基烷基化是与路易斯碱催化合作开发的。该反应由辛可尼定和原位生成的环金属化 Ir()/亚磷酰胺络合物催化,利用 α,β-不饱和羰基化合物(即香豆酸酯)的潜在烯醇化特性,在其 α 位引入烯丙基以支链选择性方式,产率中等至良好,具有良好至优异的对映选择性(高达 98 : 2 er)。
更新日期:2021-01-22
中文翻译:

铱催化对映选择性烯属C(sp2)–H烯丙基烷基化
第一个铱催化的对映选择性烯属 C(sp 2 )–H 烯丙基烷基化是与路易斯碱催化合作开发的。该反应由辛可尼定和原位生成的环金属化 Ir()/亚磷酰胺络合物催化,利用 α,β-不饱和羰基化合物(即香豆酸酯)的潜在烯醇化特性,在其 α 位引入烯丙基以支链选择性方式,产率中等至良好,具有良好至优异的对映选择性(高达 98 : 2 er)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号