当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cerium(IV) complexes with guanidinate ligands: intense colors and anomalous electronic structures
Chemical Science ( IF 7.6 ) Pub Date : 2020-12-9 , DOI: 10.1039/d0sc05193d Yusen Qiao 1, 2 , Haolin Yin 1 , Liane M Moreau 2 , Rulin Feng 3 , Robert F Higgins 1 , Brian C Manor 1 , Patrick J Carroll 1 , Corwin H Booth 2 , Jochen Autschbach 3 , Eric J Schelter 1
Chemical Science ( IF 7.6 ) Pub Date : 2020-12-9 , DOI: 10.1039/d0sc05193d Yusen Qiao 1, 2 , Haolin Yin 1 , Liane M Moreau 2 , Rulin Feng 3 , Robert F Higgins 1 , Brian C Manor 1 , Patrick J Carroll 1 , Corwin H Booth 2 , Jochen Autschbach 3 , Eric J Schelter 1
Affiliation
|
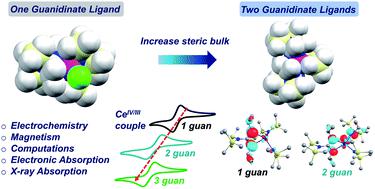
A series of cerium(IV) mixed-ligand guanidinate–amide complexes, {[(Me3Si)2NC(NiPr)2]xCeIV[N(SiMe3)2]3−x}+ (x = 0–3), was prepared by chemical oxidation of the corresponding cerium(III) complexes, where x = 1 and 2 represent novel complexes. The Ce(IV) complexes exhibited a range of intense colors, including red, black, cyan, and green. Notably, increasing the number of the guanidinate ligands from zero to three resulted in significant redshift of the absorption bands from 503 nm (2.48 eV) to 785 nm (1.58 eV) in THF. X-ray absorption near edge structure (XANES) spectra indicated increasing f occupancy (nf) with more guanidinate ligands, and revealed the multiconfigurational ground states for all Ce(IV) complexes. Cyclic voltammetry experiments demonstrated less stabilization of the Ce(IV) oxidation state with more guanidinate ligands. Moreover, the Ce(IV) tris(guanidinate) complex exhibited temperature independent paramagnetism (TIP) arising from the small energy gap between the ground- and excited states with considerable magnetic moments. Computational analysis suggested that the origin of the low energy absorption bands was a charge transfer between guanidinate π orbitals that were close in energy to the unoccupied Ce 4f orbitals. However, the incorporation of sterically hindered guanidinate ligands inhibited optimal overlaps between Ce 5d and ligand N 2p orbitals. As a result, there was an overall decrease of ligand-to-metal donation and a less stabilized Ce(IV) oxidation state, while at the same time, more of the donated electron density ended up in the 4f shell. The results indicate that incorporating guanidinate ligands into Ce(IV) complexes gives rise to intense charge transfer bands and noteworthy electronic structures, providing insights into the stabilization of tetravalent lanthanide oxidation states.
中文翻译:

具有胍基配体的铈 (IV) 配合物:浓烈的颜色和异常的电子结构
一系列铈( IV )混合配体胍盐-酰胺络合物,{[(Me 3 Si) 2 NC(N i Pr) 2 ] x Ce IV [N(SiMe 3 ) 2 ] 3− x } + ( x = 0-3),是通过化学氧化相应的铈(III)配合物制备的,其中x = 1和2代表新型配合物。Ce( IV)复合物表现出一系列强烈的颜色,包括红色、黑色、青色和绿色。值得注意的是,将胍基配体的数量从零增加到三个会导致 THF 中吸收带从 503 nm (2.48 eV) 显着红移到 785 nm (1.58 eV)。X射线吸收近边结构(XANES)光谱表明,更多的胍配体增加了f占有率(n f ),并揭示了所有Ce( IV )配合物的多构型基态。循环伏安实验表明,更多的胍配体对 Ce( IV ) 氧化态的稳定性较差。此外,Ce( IV)三(胍)配合物表现出与温度无关的顺磁性(TIP),这是由于具有相当大磁矩的基态和激发态之间的小能隙引起的。计算分析表明,低能量吸收带的起源是能量接近未占据的 Ce 4f 轨道的胍 π 轨道之间的电荷转移。然而,空间位阻胍酸配体的掺入抑制了Ce 5d 和配体N 2p 轨道之间的最佳重叠。结果,配体对金属的捐赠总体减少,Ce( IV )氧化态稳定性降低,同时更多的捐赠电子密度最终出现在 4f 壳层中。结果表明,将胍基配体掺入 Ce( IV)配合物产生强烈的电荷转移带和值得注意的电子结构,为四价镧系元素氧化态的稳定提供了见解。
更新日期:2021-01-22
中文翻译:

具有胍基配体的铈 (IV) 配合物:浓烈的颜色和异常的电子结构
一系列铈( IV )混合配体胍盐-酰胺络合物,{[(Me 3 Si) 2 NC(N i Pr) 2 ] x Ce IV [N(SiMe 3 ) 2 ] 3− x } + ( x = 0-3),是通过化学氧化相应的铈(III)配合物制备的,其中x = 1和2代表新型配合物。Ce( IV)复合物表现出一系列强烈的颜色,包括红色、黑色、青色和绿色。值得注意的是,将胍基配体的数量从零增加到三个会导致 THF 中吸收带从 503 nm (2.48 eV) 显着红移到 785 nm (1.58 eV)。X射线吸收近边结构(XANES)光谱表明,更多的胍配体增加了f占有率(n f ),并揭示了所有Ce( IV )配合物的多构型基态。循环伏安实验表明,更多的胍配体对 Ce( IV ) 氧化态的稳定性较差。此外,Ce( IV)三(胍)配合物表现出与温度无关的顺磁性(TIP),这是由于具有相当大磁矩的基态和激发态之间的小能隙引起的。计算分析表明,低能量吸收带的起源是能量接近未占据的 Ce 4f 轨道的胍 π 轨道之间的电荷转移。然而,空间位阻胍酸配体的掺入抑制了Ce 5d 和配体N 2p 轨道之间的最佳重叠。结果,配体对金属的捐赠总体减少,Ce( IV )氧化态稳定性降低,同时更多的捐赠电子密度最终出现在 4f 壳层中。结果表明,将胍基配体掺入 Ce( IV)配合物产生强烈的电荷转移带和值得注意的电子结构,为四价镧系元素氧化态的稳定提供了见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号