当前位置:
X-MOL 学术
›
Nano Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanomics Biomarker for Cancer Cells Unidentifiable through Morphology and Elastic Modulus
Nano Letters ( IF 9.6 ) Pub Date : 2021-01-21 , DOI: 10.1021/acs.nanolett.1c00003 Hongxin Wang 1 , Han Zhang 1 , Bo Da 2 , Dabao Lu 2 , Ryo Tamura 3 , Kenta Goto 4 , Ikumu Watanabe 5 , Daisuke Fujita 1 , Nobutaka Hanagata 6 , Junko Kano 7 , Tomoki Nakagawa 7 , Masayuki Noguchi 7
Nano Letters ( IF 9.6 ) Pub Date : 2021-01-21 , DOI: 10.1021/acs.nanolett.1c00003 Hongxin Wang 1 , Han Zhang 1 , Bo Da 2 , Dabao Lu 2 , Ryo Tamura 3 , Kenta Goto 4 , Ikumu Watanabe 5 , Daisuke Fujita 1 , Nobutaka Hanagata 6 , Junko Kano 7 , Tomoki Nakagawa 7 , Masayuki Noguchi 7
Affiliation
|
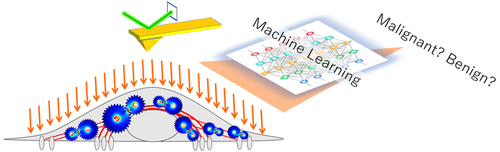
Cellular mechanical properties are potential cancer biomarkers used for objective cytology to replace the current subjective method relying on cytomorphology. However, heterogeneity among intra/intercellular mechanics and the interplay between cytoskeletal prestress and elastic modulus obscured the difference detectable between malignant and benign cells. In this work, we collected high density nanoscale prestress and elastic modulus data from a single cell by AFM indentation to generate a cellular mechanome. Such high dimensional mechanome data was used to train a malignancy classifier through machine learning. The classifier was tested on 340 single cells of various origins, malignancy, and degrees of similarity in morphology and elastic modulus. The classifier showed instrument-independent robustness and classification accuracy of 89% with an AUC-ROC value of 93%. A signal-to-noise ratio 8 times that of the human-cytologist-based morphological method was also demonstrated, in differentiating precancerous hyperplasia cells from normal cells derived from the same lung cancer patient.
中文翻译:

通过形态学和弹性模量无法识别的癌细胞的机电学生物标记
细胞力学性质是用于客观细胞学的潜在癌症生物标志物,以取代当前依赖于细胞形态学的主观方法。然而,细胞内/细胞间机制之间的异质性以及细胞骨架预应力与弹性模量之间的相互作用掩盖了恶性和良性细胞之间可检测到的差异。在这项工作中,我们通过AFM压痕从单个细胞中收集了高密度纳米级预应力和弹性模量数据,以生成细胞力学。这种高维力学数据用于通过机器学习训练恶性分类器。在340个具有不同起源,恶性以及形态和弹性模量相似程度的单细胞上测试了分类器。分类器显示出89%的独立于仪器的鲁棒性和分类准确度,AUC-ROC值为93%。还证实了信噪比是基于人类细胞学的形态学方法的8倍,可将癌前增生细胞与同一肺癌患者的正常细胞区分开。
更新日期:2021-02-10
中文翻译:

通过形态学和弹性模量无法识别的癌细胞的机电学生物标记
细胞力学性质是用于客观细胞学的潜在癌症生物标志物,以取代当前依赖于细胞形态学的主观方法。然而,细胞内/细胞间机制之间的异质性以及细胞骨架预应力与弹性模量之间的相互作用掩盖了恶性和良性细胞之间可检测到的差异。在这项工作中,我们通过AFM压痕从单个细胞中收集了高密度纳米级预应力和弹性模量数据,以生成细胞力学。这种高维力学数据用于通过机器学习训练恶性分类器。在340个具有不同起源,恶性以及形态和弹性模量相似程度的单细胞上测试了分类器。分类器显示出89%的独立于仪器的鲁棒性和分类准确度,AUC-ROC值为93%。还证实了信噪比是基于人类细胞学的形态学方法的8倍,可将癌前增生细胞与同一肺癌患者的正常细胞区分开。











































 京公网安备 11010802027423号
京公网安备 11010802027423号