当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Use of Imidazo[1,5‐a]pyridin‐3‐ylidene as a Platform for Metal‐Imidazole Cooperative Catalysis: Silver‐Catalyzed Cyclization of Alkyne‐Tethered Carboxylic Acids
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2021-01-21 , DOI: 10.1002/adsc.202001515 Vishal Kumar Rawat 1 , Kosuke Higashida 1 , Masaya Sawamura 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2021-01-21 , DOI: 10.1002/adsc.202001515 Vishal Kumar Rawat 1 , Kosuke Higashida 1 , Masaya Sawamura 1
Affiliation
|
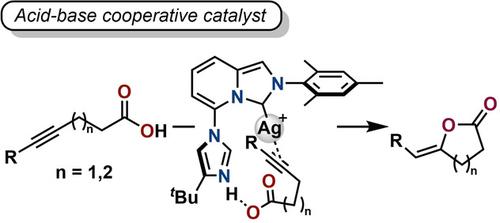
Silver complexes with 5‐(4‐(tert‐butyl)‐1H‐imidazol‐1‐yl)‐imidazo[1,5‐a]pyridin‐3‐ylidene ligands were synthesized as metal‐imidazole acid‐base cooperative catalysts. Single crystal XRD analysis revealed that the silver atom was located in the vicinity of the imidazole ring and that cationic silver complexes formed dimers through coordination between the silver metal and the imidazole pendant. These cationic silver complexes served as catalysts for cyclization of alkyne‐tethered carboxylic acids. NMR experiments indicated that the dimeric cationic silver complex dissociated to a monomer upon protonation of the imidazole moiety, resulting in coordination of an acetonitrile to the silver atom. DFT calculations supported the acid‐base cooperative action of the silver‐imidazole for the efficient alkyne‐carboxylic acid cyclization.
中文翻译:

咪唑并[1,5-a]吡啶-3-亚烷基用作金属-咪唑协同催化的平台:炔烃系羧酸的银催化环化
具有5-(4- (叔丁基)-1 H-咪唑-1-基)-咪唑的银络合物[1,5- a]吡啶-3-亚烷基配体被合成为金属咪唑酸碱协同催化剂。单晶XRD分析表明银原子位于咪唑环附近,并且阳离子银络合物通过银金属和咪唑侧基之间的配位形成二聚体。这些阳离子银络合物可作为炔烃系羧酸环化的催化剂。NMR实验表明,二咪唑阳离子银配合物在咪唑部分质子化时解离为单体,从而导致乙腈与银原子的配位。DFT计算结果支持了咪唑银的酸碱协同作用,从而有效地进行了炔-羧酸环化反应。
更新日期:2021-03-16
中文翻译:

咪唑并[1,5-a]吡啶-3-亚烷基用作金属-咪唑协同催化的平台:炔烃系羧酸的银催化环化
具有5-(4- (叔丁基)-1 H-咪唑-1-基)-咪唑的银络合物[1,5- a]吡啶-3-亚烷基配体被合成为金属咪唑酸碱协同催化剂。单晶XRD分析表明银原子位于咪唑环附近,并且阳离子银络合物通过银金属和咪唑侧基之间的配位形成二聚体。这些阳离子银络合物可作为炔烃系羧酸环化的催化剂。NMR实验表明,二咪唑阳离子银配合物在咪唑部分质子化时解离为单体,从而导致乙腈与银原子的配位。DFT计算结果支持了咪唑银的酸碱协同作用,从而有效地进行了炔-羧酸环化反应。



























 京公网安备 11010802027423号
京公网安备 11010802027423号