Applied Geochemistry ( IF 3.1 ) Pub Date : 2021-01-21 , DOI: 10.1016/j.apgeochem.2021.104893 Fuwei Sun , Tianhu Chen , Xuehua Zou , Haibo Liua , Ziyang Chu , Daobing Shu , Hanlin Wang , Fangju Huang , Dong Chen
|
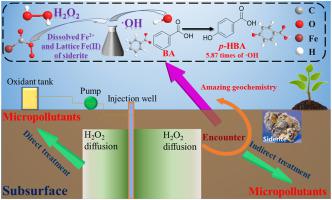
Siderite is a conventional mineral of sedimentary rock and can activate H2O2 to produce hydroxyl radicals (·OH). Bisphenol A (BPA), 2,4-dichlorophenoxyacetic acid (2,4-D), and sodium sulfadiazine are selected as typical micropollutants to be degraded in a siderite-activated H2O2 system. To determine the influence of solution chemistry on siderite-activated Fenton-like reactions, the amount of ·OH was quantified by using benzoic acid as a probe molecule, and then, kinetic fitting was used to analyze the reaction rate. The results showed that in the range of pH values from 3 to 9, the dissolved Fe2+ and the lattice Fe(Ⅱ) of siderite activate H2O2 to generate ·OH. An increase in siderite dosage and H2O2 concentration favored the generation of ·OH, but higher siderite dosage and H2O2 concentration suppressed the production rate. The inhibition order of anions on the generation of ·OH was confirmed as follows > > . Little difference was observed in the presence of Ca2+ and Mg2+, while the presence of Mn2+ significantly promoted the production of ·OH. The suppression of humic acid (HA) became more serious as the HA concentration increased. Cycling experiments proved that the system could still produce ·OH in four cycles, although the production rate experienced a slight decrease. The experimental results suggested the role of siderite in the degradation of organic pollution in the process of in-situ soil and groundwater remediation.
中文翻译:

H 2 O 2遇到菱铁矿时产生羟基自由基的定量分析:动力学和参数影响
菱铁矿是沉积岩中的常规矿物,可以活化H 2 O 2产生羟自由基(·OH)。选择双酚A(BPA),2,4-二氯苯氧基乙酸(2,4-D)和磺胺嘧啶钠作为典型的微污染物,可在菱铁矿活化的H 2 O 2系统中降解。为了确定溶液化学性质对菱铁矿活化的Fenton样反应的影响,通过使用苯甲酸作为探针分子定量·OH的量,然后使用动力学拟合分析反应速率。结果表明,在pH值为3〜9的范围内,溶解的Fe 2+和菱铁矿的晶格Fe(Ⅱ)激活了H 2 O 2。生成·OH。菱铁矿剂量和H 2 O 2浓度的增加有利于·OH的生成,但是较高的菱铁矿剂量和H 2 O 2浓度抑制了生产率。阴离子对·OH生成的抑制顺序如下 > > 。Ca 2+和Mg 2+的存在几乎没有差异,而Mn 2+的存在显着促进了·OH的产生。腐殖酸(HA)的抑制随着HA浓度的增加而变得更加严重。循环实验证明,尽管产率略有下降,该系统仍可以在四个循环中产生·OH。实验结果表明,菱铁矿在原位土壤和地下水修复过程中对有机污染的降解具有重要作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号