当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Anion effects on Li ion transference number and dynamic ion correlations in glyme–Li salt equimolar mixtures
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2021-1-15 , DOI: 10.1039/d0cp06381a Keisuke Shigenobu 1, 2, 3, 4 , Masayuki Shibata 4, 5, 6, 7 , Kaoru Dokko 1, 2, 3, 4, 8 , Masayoshi Watanabe 2, 3, 4, 8, 9 , Kenta Fujii 4, 5, 6, 7 , Kazuhide Ueno 1, 2, 3, 4, 8
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2021-1-15 , DOI: 10.1039/d0cp06381a Keisuke Shigenobu 1, 2, 3, 4 , Masayuki Shibata 4, 5, 6, 7 , Kaoru Dokko 1, 2, 3, 4, 8 , Masayoshi Watanabe 2, 3, 4, 8, 9 , Kenta Fujii 4, 5, 6, 7 , Kazuhide Ueno 1, 2, 3, 4, 8
Affiliation
|
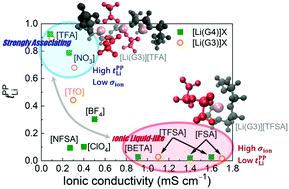
To achieve single-ion conducting liquid electrolytes for the rapid charge and discharge of Li secondary batteries, improvement in the Li+ transference number of the electrolytes is integral. Few studies have established a feasible design for achieving Li+ transference numbers approaching unity in liquid electrolytes consisting of low-molecular-weight salts and solvents. Previously, we studied the effects of Li+–solvent interactions on the Li+ transference number in glyme- and sulfolane-based molten Li salt solvates and clarified the relationship between this transference number and correlated ion motions. In this study, to deepen our insight into the design principles of single-ion conducting liquid electrolytes, we focused on the effects of Li+–anion interactions on Li ion transport in glyme–Li salt equimolar mixtures with different counter anions. Interestingly, the equimolar triglyme (G3)–lithium trifluoroacetate (Li[TFA]) mixture ([Li(G3)][TFA]) demonstrated a high Li+ transference number, estimated via the potentiostatic polarization method (tPPLi = 0.90). Dynamic ion correlation studies suggested that the high tPPLi could be mainly ascribed to the strongly coupled Li+–anion motions in the electrolytes. Furthermore, high-energy X-ray total scattering measurements combined with all-atom molecular dynamics simulations showed that Li+ ions and [TFA] anions aggregated into ionic clusters with a relatively long-range ion-ordered structure. Therefore, the collective motions of the Li ions and anions in the form of highly aggregated ion clusters, which likely diminish rather than enhance ionic conductivity, play a significant role in achieving high tPPLi in liquid electrolytes. Based on the dynamic ion correlations, a potential design approach is discussed to accomplish single-ion conducting liquid electrolytes with high ionic conductivity.
中文翻译:

阴离子对甘氨酸-锂盐等摩尔混合物中锂离子迁移数和动态离子相关性的影响
为了实现用于锂二次电池的快速充电和放电的单离子导电液体电解质,电解质的Li +迁移数的提高是不可或缺的。很少有研究建立可行的设计,以在由低分子量盐和溶剂组成的液体电解质中实现接近统一的Li +转移数。以前,我们研究了Li +-溶剂相互作用对Li +的影响基于甘醇二甲醚和环丁砜的熔融锂盐溶剂化物中的离子转移数,并阐明了该转移数与相关离子运动之间的关系。在本研究中,为了加深对单离子导电液体电解质设计原理的了解,我们重点研究了Li +-阴离子相互作用对具有不同抗衡阴离子的甘氨酸-Li盐等摩尔混合物中Li离子迁移的影响。有趣的是,等摩尔三甘醇二甲醚(G3)-三氟乙酸锂(Li [TFA])混合物([Li(G3)] [TFA])表现出高的Li +转移数,通过恒电位极化法估算(t PP Li = 0.90) 。动态离子相关性研究表明,高t PPLi可能主要归因于电解质中Li +-阴离子的强耦合运动。此外,高能X射线总散射测量与全原子分子动力学模拟相结合显示,Li +离子和[TFA]阴离子聚集成具有相对长距离离子有序结构的离子簇。因此,锂离子和阴离子以高度聚集的离子簇的形式发生的集体运动可能会降低而不是增强离子电导率,在实现高t PP Li方面起着重要作用。在液体电解质中。基于动态离子相关性,讨论了一种潜在的设计方法,以完成具有高离子电导率的单离子导电液体电解质。
更新日期:2021-01-21
中文翻译:

阴离子对甘氨酸-锂盐等摩尔混合物中锂离子迁移数和动态离子相关性的影响
为了实现用于锂二次电池的快速充电和放电的单离子导电液体电解质,电解质的Li +迁移数的提高是不可或缺的。很少有研究建立可行的设计,以在由低分子量盐和溶剂组成的液体电解质中实现接近统一的Li +转移数。以前,我们研究了Li +-溶剂相互作用对Li +的影响基于甘醇二甲醚和环丁砜的熔融锂盐溶剂化物中的离子转移数,并阐明了该转移数与相关离子运动之间的关系。在本研究中,为了加深对单离子导电液体电解质设计原理的了解,我们重点研究了Li +-阴离子相互作用对具有不同抗衡阴离子的甘氨酸-Li盐等摩尔混合物中Li离子迁移的影响。有趣的是,等摩尔三甘醇二甲醚(G3)-三氟乙酸锂(Li [TFA])混合物([Li(G3)] [TFA])表现出高的Li +转移数,通过恒电位极化法估算(t PP Li = 0.90) 。动态离子相关性研究表明,高t PPLi可能主要归因于电解质中Li +-阴离子的强耦合运动。此外,高能X射线总散射测量与全原子分子动力学模拟相结合显示,Li +离子和[TFA]阴离子聚集成具有相对长距离离子有序结构的离子簇。因此,锂离子和阴离子以高度聚集的离子簇的形式发生的集体运动可能会降低而不是增强离子电导率,在实现高t PP Li方面起着重要作用。在液体电解质中。基于动态离子相关性,讨论了一种潜在的设计方法,以完成具有高离子电导率的单离子导电液体电解质。









































 京公网安备 11010802027423号
京公网安备 11010802027423号