当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Access to 12-Membered Cyclic ortho,meta-Diarylheptanoids: Total Synthesis of Actinidione via Isomyricanone
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-01-21 , DOI: 10.1021/acs.joc.0c02489 Paul Massé 1 , Sabine Choppin 1 , Lucia Chiummiento 2 , Françoise Colobert 1 , Gilles Hanquet 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-01-21 , DOI: 10.1021/acs.joc.0c02489 Paul Massé 1 , Sabine Choppin 1 , Lucia Chiummiento 2 , Françoise Colobert 1 , Gilles Hanquet 1
Affiliation
|
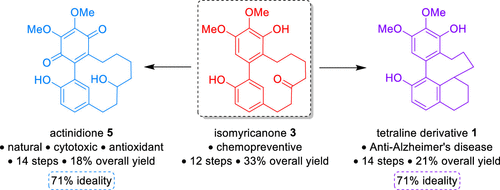
We describe herein the first access to 12-membered cyclic[7,0]ortho,meta-diarylheptanoids. The key features of the synthesis include both a Suzuki–Miyaura coupling and a ring closing metathesis. Actinidione, a promising natural product, along with a bioactive tetracyclic derivative were obtained in 14 steps for the first time from cheap commercially available substrates with an overall yield of 18–21%. Our modus operandi complies with the principles of the synthesis ideality by using notably strategic reactions.
中文翻译:

进入12成员的环状邻位,间-二芳基庚烷类化合物:通过异麦芽酮酮合成放线菌酮
我们在此描述〜12元环[7,0]第一接入邻,间位-diarylheptanoids。合成的关键特征包括Suzuki-Miyaura偶联和闭环易位。放线菌酮,一种有前途的天然产物,以及具有生物活性的四环衍生物,是第14步首次从廉价的市售底物中获得,总收率为18-21%。我们的作案手法通过使用显着的战略反应来符合综合理想化的原则。
更新日期:2021-02-05
中文翻译:

进入12成员的环状邻位,间-二芳基庚烷类化合物:通过异麦芽酮酮合成放线菌酮
我们在此描述〜12元环[7,0]第一接入邻,间位-diarylheptanoids。合成的关键特征包括Suzuki-Miyaura偶联和闭环易位。放线菌酮,一种有前途的天然产物,以及具有生物活性的四环衍生物,是第14步首次从廉价的市售底物中获得,总收率为18-21%。我们的作案手法通过使用显着的战略反应来符合综合理想化的原则。











































 京公网安备 11010802027423号
京公网安备 11010802027423号