当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Acid-Catalyzed Hydrothiolation of gem-Difluorostyrenes to Access α,α-Difluoroalkylthioethers
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-01-20 , DOI: 10.1021/acs.joc.0c02440 Jacob P Sorrentino 1 , Douglas L Orsi 2 , Ryan A Altman 3
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-01-20 , DOI: 10.1021/acs.joc.0c02440 Jacob P Sorrentino 1 , Douglas L Orsi 2 , Ryan A Altman 3
Affiliation
|
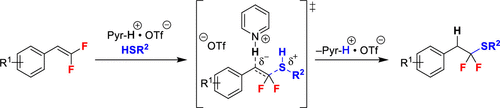
The substitution of hydrogen atoms with fluorine in bioactive molecules can greatly impact physicochemical, pharmacokinetic, and pharmacodynamic properties. However, current synthetic methods cannot readily access many fluorinated motifs, which impedes utilization of these groups. Thus, the development of new methods to introduce fluorinated functional groups is critical for developing the next generation of biological probes and therapeutic agents. The synthesis of one such substructure, the α,α-difluoroalkylthioether, typically requires specialized conditions that necessitate early-stage installation. A late-stage and convergent approach to access α,α-difluoroalkylthioethers could involve nucleophilic addition of thiols across gem-difluorostyrenes. Unfortunately, under basic conditions, nucleophilic addition to gem-difluorostyrenes generates an anionic intermediate that can undergo facile elimination of fluoride to generate α-fluorovinylthioethers. To overcome this decomposition, we herein exploit an acid-based catalyst system to facilitate simultaneous nucleophilic addition and protonation of the unstable intermediate. Ultimately, the optimized mild conditions afford the desired α,α-difluoroalkylthioethers in high selectivity and moderate to excellent yields. These α,α-difluoroalkylthioethers are less nucleophilic and more oxidatively stable relative to nonfluorinated thioethers, suggesting the potential application of this unexplored functional group in biological probes and therapeutic agents.
中文翻译:

酸催化偕二氟苯乙烯氢硫基化反应生成 α,α-二氟烷基硫醚
生物活性分子中氢原子被氟取代可以极大地影响物理化学、药代动力学和药效学特性。然而,当前的合成方法无法轻易获得许多氟化基序,这阻碍了这些基团的利用。因此,开发引入氟化官能团的新方法对于开发下一代生物探针和治疗剂至关重要。 α,α-二氟烷基硫醚这样的一种子结构的合成通常需要特殊的条件,因此需要早期安装。获取 α,α-二氟烷基硫醚的后期聚合方法可能涉及跨偕二氟苯乙烯的硫醇亲核加成。不幸的是,在碱性条件下,偕二氟苯乙烯的亲核加成会产生阴离子中间体,该阴离子中间体可以轻易消除氟化物,生成α-氟乙烯基硫醚。为了克服这种分解,我们在此利用酸基催化剂系统来促进不稳定中间体的同时亲核加成和质子化。最终,优化的温和条件以高选择性和中等至优异的收率提供了所需的 α,α-二氟烷基硫醚。与非氟化硫醚相比,这些α,α-二氟烷基硫醚的亲核性较低,氧化稳定性更高,表明这种未开发的官能团在生物探针和治疗剂中的潜在应用。
更新日期:2021-02-05
中文翻译:

酸催化偕二氟苯乙烯氢硫基化反应生成 α,α-二氟烷基硫醚
生物活性分子中氢原子被氟取代可以极大地影响物理化学、药代动力学和药效学特性。然而,当前的合成方法无法轻易获得许多氟化基序,这阻碍了这些基团的利用。因此,开发引入氟化官能团的新方法对于开发下一代生物探针和治疗剂至关重要。 α,α-二氟烷基硫醚这样的一种子结构的合成通常需要特殊的条件,因此需要早期安装。获取 α,α-二氟烷基硫醚的后期聚合方法可能涉及跨偕二氟苯乙烯的硫醇亲核加成。不幸的是,在碱性条件下,偕二氟苯乙烯的亲核加成会产生阴离子中间体,该阴离子中间体可以轻易消除氟化物,生成α-氟乙烯基硫醚。为了克服这种分解,我们在此利用酸基催化剂系统来促进不稳定中间体的同时亲核加成和质子化。最终,优化的温和条件以高选择性和中等至优异的收率提供了所需的 α,α-二氟烷基硫醚。与非氟化硫醚相比,这些α,α-二氟烷基硫醚的亲核性较低,氧化稳定性更高,表明这种未开发的官能团在生物探针和治疗剂中的潜在应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号