当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Phase Equilibrium Study of the Aqueous Solution of Propanoic Acid with Acetates at Different Temperatures
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-01-21 , DOI: 10.1021/acs.jced.0c00151 Mahboobe Behroozi 1 , Sahar Heidari 1 , Arezou Malekpour 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-01-21 , DOI: 10.1021/acs.jced.0c00151 Mahboobe Behroozi 1 , Sahar Heidari 1 , Arezou Malekpour 1
Affiliation
|
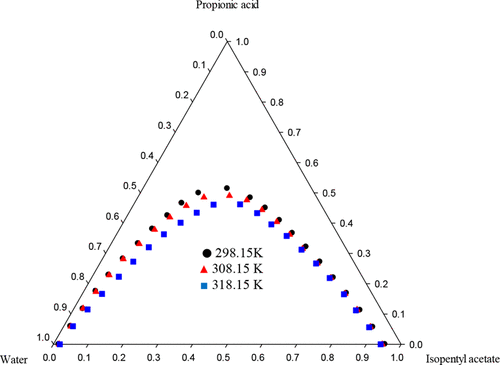
Ternary phase behavior of water + propanoic acid + 3-methylbutyl acetate and water + propanoic acid + propan-2-yl acetate ternary mixtures including solubility and tie-line data was defined at (298.15 to 318.15) K under the pressure of 83.4 ± 1 kPa. The compositions of tie-line and bimodal curves were obtained by refractive index measurements. The phase diagrams show the type-1 behavior of liquid–liquid equilibrium (LLE). The capability of the solvents for the extraction of propanoic acid from aqueous solutions was determined by distribution coefficients and separation factors. The reliability of the experimental tie-line data was ascertained by Othmer–Tobias and Hand correlation equations. The tie-line data were correlated with nonrandom two-liquid (NRTL) and universal quasi-chemical (UNIQUAC) models. The binary interaction parameters of the models were reported. Excellent agreement was observed between the experimental data and the correlated values.
中文翻译:

不同温度下丙酸与乙酸的水溶液的相平衡研究
在83.4±1的压力下,水+丙酸+乙酸3-甲基丁酯和水+丙酸+乙酸丙-2-基酯的三元混合物的三元相行为(包括溶解度和临界线数据)定义为(298.15至318.15)K千帕 连接线和双峰曲线的组成通过折射率测量获得。相图显示了液-液平衡(LLE)的1类行为。溶剂从水溶液中提取丙酸的能力由分布系数和分离系数确定。实验联系线数据的可靠性通过Othmer-Tobias和Hand相关方程式确定。联络线数据与非随机二液(NRTL)模型和通用拟化学(UNIQUAC)模型相关。报告了模型的二元相互作用参数。实验数据和相关值之间观察到极好的一致性。
更新日期:2021-02-11
中文翻译:

不同温度下丙酸与乙酸的水溶液的相平衡研究
在83.4±1的压力下,水+丙酸+乙酸3-甲基丁酯和水+丙酸+乙酸丙-2-基酯的三元混合物的三元相行为(包括溶解度和临界线数据)定义为(298.15至318.15)K千帕 连接线和双峰曲线的组成通过折射率测量获得。相图显示了液-液平衡(LLE)的1类行为。溶剂从水溶液中提取丙酸的能力由分布系数和分离系数确定。实验联系线数据的可靠性通过Othmer-Tobias和Hand相关方程式确定。联络线数据与非随机二液(NRTL)模型和通用拟化学(UNIQUAC)模型相关。报告了模型的二元相互作用参数。实验数据和相关值之间观察到极好的一致性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号