当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
High-Pressure Phase Equilibria in an Ethanol/Water Binary System: Experimental Data and Modeling
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-01-21 , DOI: 10.1021/acs.jced.0c00686 Pedro Susial Badajoz 1 , Diego García-Vera 1 , Patricia Herrera-Vega 1 , Anibal Marrero-Pérez 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-01-21 , DOI: 10.1021/acs.jced.0c00686 Pedro Susial Badajoz 1 , Diego García-Vera 1 , Patricia Herrera-Vega 1 , Anibal Marrero-Pérez 1
Affiliation
|
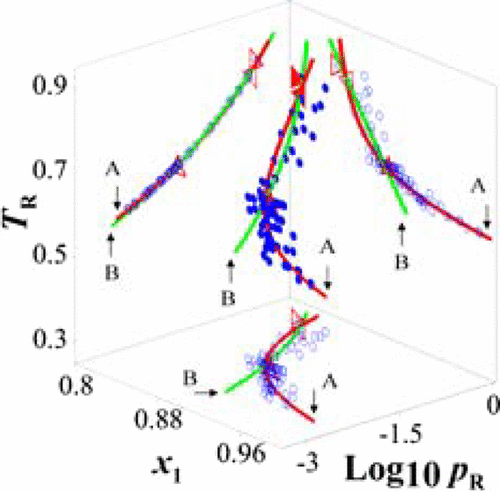
Experimental data of vapor–liquid equilibria (VLE) are reported for the binary systems: ethanol (1) + water (2) at 100, 1500, and 2000 kPa. The data were verified for thermodynamic consistency by using different low- and high-pressure tests. The following minimum azeotropes were found for the binary systems: x1,az = y1,az = 0.899 and T = 351.39 K at 100 kPa, x1,az = y1,az = 0.857 and T = 440.70 K at 1500 kPa, and x1,az = y1,az = 0.850 and T = 453.83 K at 2000 kPa. The quality of the azeotropic data obtained was established by considering the trend of the bibliographic azeotropic data. The phase behavior was modeled using the Peng–Robinson equation of state (EOS) with several mixing rules and the perturbed-chain statistical associating fluid theory. The experimental data were satisfactorily correlated and a qualitative agreement with these predictive models was observed.
中文翻译:

乙醇/水二元系统中的高压相平衡:实验数据和建模
报告了二元系统的气液平衡(VLE)实验数据:乙醇(1)+水(2)在100、1500和2000 kPa时。通过使用不同的低压和高压测试,验证了数据的热力学一致性。结果发现以下的最低共沸物为二元系统:X 1,AZ = ÿ 1,AZ = 0.899和Ť = 351.39处的K 100千帕,X 1,AZ = ÿ 1,AZ = 0.857和Ť = 440.70处的K 1500千帕,并且x 1,az = y 1,az = 0.850和T在2000 kPa时为453.83 K. 通过考虑书目共沸数据的趋势来确定获得的共沸数据的质量。使用彭-罗宾逊状态方程(EOS),几个混合规则和扰动链统计关联流体理论对相行为进行建模。实验数据令人满意地相关,并且观察到与这些预测模型的定性一致性。
更新日期:2021-02-11
中文翻译:

乙醇/水二元系统中的高压相平衡:实验数据和建模
报告了二元系统的气液平衡(VLE)实验数据:乙醇(1)+水(2)在100、1500和2000 kPa时。通过使用不同的低压和高压测试,验证了数据的热力学一致性。结果发现以下的最低共沸物为二元系统:X 1,AZ = ÿ 1,AZ = 0.899和Ť = 351.39处的K 100千帕,X 1,AZ = ÿ 1,AZ = 0.857和Ť = 440.70处的K 1500千帕,并且x 1,az = y 1,az = 0.850和T在2000 kPa时为453.83 K. 通过考虑书目共沸数据的趋势来确定获得的共沸数据的质量。使用彭-罗宾逊状态方程(EOS),几个混合规则和扰动链统计关联流体理论对相行为进行建模。实验数据令人满意地相关,并且观察到与这些预测模型的定性一致性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号