当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Measurement and PC-SAFT Modeling of the Solubility of Gallic Acid in Aqueous Mixtures of Deep Eutectic Solvents
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-01-20 , DOI: 10.1021/acs.jced.0c00784 Bruno Sepúlveda-Orellana 1 , Nicolás F. Gajardo-Parra 1, 2 , Hoang T. Do 2 , José R. Pérez-Correa 1 , Christoph Held 2 , Gabriele Sadowski 2 , Roberto I. Canales 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-01-20 , DOI: 10.1021/acs.jced.0c00784 Bruno Sepúlveda-Orellana 1 , Nicolás F. Gajardo-Parra 1, 2 , Hoang T. Do 2 , José R. Pérez-Correa 1 , Christoph Held 2 , Gabriele Sadowski 2 , Roberto I. Canales 1
Affiliation
|
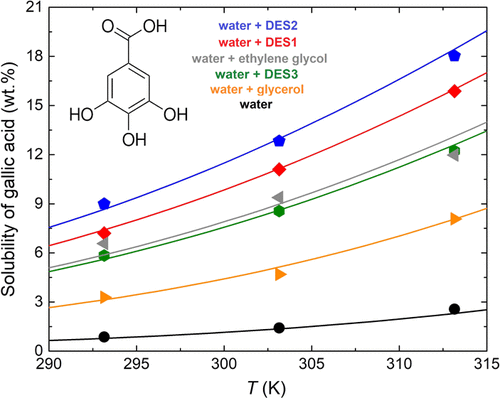
Deep eutectic solvents have appeared as potential solvents for improving the extraction of polyphenols from vegetable or fruit matrixes. Since gallic acid is abundant in these sources, it is considered as a typical standard for quantifying their total polyphenol content after extraction with solvents. However, there are no extensive studies on the solubility behavior of gallic acid in different solvents or deep eutectic solvents. Thus, in this work, the solubility of gallic acid is measured in pure water; aqueous solutions of different hydrogen bond donors such as ethylene glycol, levulinic acid, and glycerol; and aqueous mixtures of deep eutectic solvents using choline chloride as the hydrogen bond acceptor and ethylene glycol, levulinic acid, and glycerol as the hydrogen bond donors. All of the measurements were performed at 293.15, 303.15, and 313.15 K and at 101.3 kPa and were validated by comparing the solubility of gallic acid in water from the literature. Results suggest that a 50 wt % aqueous solution of deep eutectic solvent based on ethylene glycol or glycerol improves the gallic acid solubility compared with a 50 wt % aqueous solution of its corresponding hydrogen bond donor. The deep eutectic solvent containing levulinic acid acts as the best aqueous mixture for gallic acid dissolution. Nondissolved gallic acid was measured after equilibrium using powder X-ray diffraction, showing that its structure does not change upon mixing with all of the liquid mixtures. All of the solid–liquid equilibrium results were accurately modeled with perturbed-chain statistical associating fluid theory (PC-SAFT).
中文翻译:

高共晶溶剂在水混合物中没食子酸溶解度的测量和PC-SAFT建模
深共晶溶剂似乎已成为改善从蔬菜或水果基质中提取多酚的潜在溶剂。由于没食子酸在这些来源中含量很高,因此被认为是定量用溶剂萃取后其总多酚含量的典型标准。但是,关于没食子酸在不同溶剂或深共熔溶剂中的溶解行为,尚无广泛研究。因此,在这项工作中,测量了没食子酸在纯水中的溶解度;不同氢键供体的水溶液,例如乙二醇,乙酰丙酸和甘油;以及使用氯化胆碱作为氢键受体,乙二醇,乙酰丙酸和甘油作为氢键供体的深共晶溶剂的水性混合物。所有测量均在293.15、303.15,通过比较文献中的没食子酸在水中的溶解度,验证了313.15 K和101.3 kPa时的效价。结果表明,与50wt%的相应氢键供体的水溶液相比,基于乙二醇或甘油的50wt%的深共晶溶剂的水溶液改善了没食子酸的溶解性。含有乙酰丙酸的深共熔溶剂是没食子酸溶解的最佳水性混合物。平衡后,使用粉末X射线衍射测量未溶解的没食子酸,表明其结构在与所有液体混合物混合后不会改变。所有的固液平衡结果均采用扰动链统计缔合流体理论(PC-SAFT)精确建模。通过比较文献中没食子酸在水中的溶解度来验证3 kPa和。结果表明,与50wt%的相应氢键供体的水溶液相比,基于乙二醇或甘油的50wt%的深共晶溶剂的水溶液改善了没食子酸的溶解性。含有乙酰丙酸的深共熔溶剂是没食子酸溶解的最佳水性混合物。平衡后,使用粉末X射线衍射测量未溶解的没食子酸,表明其结构在与所有液体混合物混合后不会改变。所有的固液平衡结果均采用扰动链统计缔合流体理论(PC-SAFT)精确建模。通过比较文献中没食子酸在水中的溶解度来验证3 kPa和。结果表明,与50wt%的相应氢键供体的水溶液相比,基于乙二醇或甘油的50wt%的深共晶溶剂的水溶液改善了没食子酸的溶解性。含有乙酰丙酸的深共熔溶剂是没食子酸溶解的最佳水性混合物。平衡后,使用粉末X射线衍射测量未溶解的没食子酸,表明其结构在与所有液体混合物混合后不会改变。所有的固液平衡结果均采用扰动链统计缔合流体理论(PC-SAFT)精确建模。结果表明,与50wt%的相应氢键供体的水溶液相比,基于乙二醇或甘油的50wt%的深共晶溶剂的水溶液改善了没食子酸的溶解性。含有乙酰丙酸的深共熔溶剂是没食子酸溶解的最佳水性混合物。平衡后,使用粉末X射线衍射测量未溶解的没食子酸,表明其结构在与所有液体混合物混合后不会改变。所有的固液平衡结果均采用扰动链统计缔合流体理论(PC-SAFT)精确建模。结果表明,与50wt%的相应氢键供体的水溶液相比,基于乙二醇或甘油的50wt%的深共晶溶剂的水溶液改善了没食子酸的溶解性。含有乙酰丙酸的深共熔溶剂是没食子酸溶解的最佳水性混合物。平衡后,使用粉末X射线衍射测量未溶解的没食子酸,表明其结构在与所有液体混合物混合后不会改变。所有的固液平衡结果均采用扰动链统计缔合流体理论(PC-SAFT)精确建模。含有乙酰丙酸的深共熔溶剂是没食子酸溶解的最佳水性混合物。平衡后,使用粉末X射线衍射测量未溶解的没食子酸,表明其结构在与所有液体混合物混合后不会改变。所有的固液平衡结果均采用扰动链统计缔合流体理论(PC-SAFT)精确建模。含有乙酰丙酸的深共熔溶剂是没食子酸溶解的最佳水性混合物。平衡后,使用粉末X射线衍射测量未溶解的没食子酸,表明其结构在与所有液体混合物混合后不会改变。所有的固液平衡结果均采用扰动链统计缔合流体理论(PC-SAFT)精确建模。
更新日期:2021-02-11
中文翻译:

高共晶溶剂在水混合物中没食子酸溶解度的测量和PC-SAFT建模
深共晶溶剂似乎已成为改善从蔬菜或水果基质中提取多酚的潜在溶剂。由于没食子酸在这些来源中含量很高,因此被认为是定量用溶剂萃取后其总多酚含量的典型标准。但是,关于没食子酸在不同溶剂或深共熔溶剂中的溶解行为,尚无广泛研究。因此,在这项工作中,测量了没食子酸在纯水中的溶解度;不同氢键供体的水溶液,例如乙二醇,乙酰丙酸和甘油;以及使用氯化胆碱作为氢键受体,乙二醇,乙酰丙酸和甘油作为氢键供体的深共晶溶剂的水性混合物。所有测量均在293.15、303.15,通过比较文献中的没食子酸在水中的溶解度,验证了313.15 K和101.3 kPa时的效价。结果表明,与50wt%的相应氢键供体的水溶液相比,基于乙二醇或甘油的50wt%的深共晶溶剂的水溶液改善了没食子酸的溶解性。含有乙酰丙酸的深共熔溶剂是没食子酸溶解的最佳水性混合物。平衡后,使用粉末X射线衍射测量未溶解的没食子酸,表明其结构在与所有液体混合物混合后不会改变。所有的固液平衡结果均采用扰动链统计缔合流体理论(PC-SAFT)精确建模。通过比较文献中没食子酸在水中的溶解度来验证3 kPa和。结果表明,与50wt%的相应氢键供体的水溶液相比,基于乙二醇或甘油的50wt%的深共晶溶剂的水溶液改善了没食子酸的溶解性。含有乙酰丙酸的深共熔溶剂是没食子酸溶解的最佳水性混合物。平衡后,使用粉末X射线衍射测量未溶解的没食子酸,表明其结构在与所有液体混合物混合后不会改变。所有的固液平衡结果均采用扰动链统计缔合流体理论(PC-SAFT)精确建模。通过比较文献中没食子酸在水中的溶解度来验证3 kPa和。结果表明,与50wt%的相应氢键供体的水溶液相比,基于乙二醇或甘油的50wt%的深共晶溶剂的水溶液改善了没食子酸的溶解性。含有乙酰丙酸的深共熔溶剂是没食子酸溶解的最佳水性混合物。平衡后,使用粉末X射线衍射测量未溶解的没食子酸,表明其结构在与所有液体混合物混合后不会改变。所有的固液平衡结果均采用扰动链统计缔合流体理论(PC-SAFT)精确建模。结果表明,与50wt%的相应氢键供体的水溶液相比,基于乙二醇或甘油的50wt%的深共晶溶剂的水溶液改善了没食子酸的溶解性。含有乙酰丙酸的深共熔溶剂是没食子酸溶解的最佳水性混合物。平衡后,使用粉末X射线衍射测量未溶解的没食子酸,表明其结构在与所有液体混合物混合后不会改变。所有的固液平衡结果均采用扰动链统计缔合流体理论(PC-SAFT)精确建模。结果表明,与50wt%的相应氢键供体的水溶液相比,基于乙二醇或甘油的50wt%的深共晶溶剂的水溶液改善了没食子酸的溶解性。含有乙酰丙酸的深共熔溶剂是没食子酸溶解的最佳水性混合物。平衡后,使用粉末X射线衍射测量未溶解的没食子酸,表明其结构在与所有液体混合物混合后不会改变。所有的固液平衡结果均采用扰动链统计缔合流体理论(PC-SAFT)精确建模。含有乙酰丙酸的深共熔溶剂是没食子酸溶解的最佳水性混合物。平衡后,使用粉末X射线衍射测量未溶解的没食子酸,表明其结构在与所有液体混合物混合后不会改变。所有的固液平衡结果均采用扰动链统计缔合流体理论(PC-SAFT)精确建模。含有乙酰丙酸的深共熔溶剂是没食子酸溶解的最佳水性混合物。平衡后,使用粉末X射线衍射测量未溶解的没食子酸,表明其结构在与所有液体混合物混合后不会改变。所有的固液平衡结果均采用扰动链统计缔合流体理论(PC-SAFT)精确建模。











































 京公网安备 11010802027423号
京公网安备 11010802027423号