当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thermophysical Properties of Mixtures of 1-Ethyl-3-methylimidazolium Methylsulfate or 1-Ethyl-3-methylimidazolium Thiocyanate with Alcohols
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-01-20 , DOI: 10.1021/acs.jced.0c00801 Estela Lladosa 1 , Sonia Loras 1 , Helena Poy 1 , Lohengrin Caballero 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-01-20 , DOI: 10.1021/acs.jced.0c00801 Estela Lladosa 1 , Sonia Loras 1 , Helena Poy 1 , Lohengrin Caballero 1
Affiliation
|
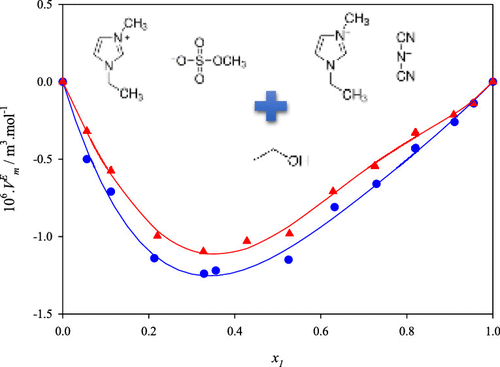
In the present paper, densities, speeds of sound, and viscosities of four binary mixtures containing the ionic liquids 1-ethyl-3-methylimidazolium methylsulfate and 1-ethyl-3-methylimidazolium thiocyanate mixed with ethanol or 1-propanol were measured at 101.3 kPa in the range of (283.15–323.15) K along the whole composition range. From these experimental data, the excess molar volume and the excess isentropic compressibility of pure components and mixtures were calculated and satisfactorily fitted using the Redlich–Kister polynomial equation. It can be confirmed that the organic compounds have a strong effect on the thermophysical properties of the studied ionic liquids. An increase in the alkyl chain length of 1-alcohol resulted in an increase in the excess deviation of excess molar volume.
中文翻译:

1-乙基-3-甲基咪唑鎓甲基硫酸盐或1-乙基-3-甲基咪唑鎓硫氰酸盐与醇的混合物的热物理性质
在本文中,在101.3 kPa下测量了包含离子液体1-乙基-3-甲基咪唑甲基硫酸盐和1-乙基-3-甲基咪唑硫氰酸盐与乙醇或1-丙醇的离子液体的四种二元混合物的密度,声速和粘度。整个合成范围内的(283.15–323.15)K范围内。从这些实验数据中,可以计算出纯组分和混合物的过量摩尔体积和等熵可压缩性,并使用Redlich-Kister多项式方程令人满意地拟合。可以证实,有机化合物对所研究的离子液体的热物理性质具有很强的影响。1-醇的烷基链长度的增加导致过量摩尔体积的过量偏差的增加。
更新日期:2021-02-11
中文翻译:

1-乙基-3-甲基咪唑鎓甲基硫酸盐或1-乙基-3-甲基咪唑鎓硫氰酸盐与醇的混合物的热物理性质
在本文中,在101.3 kPa下测量了包含离子液体1-乙基-3-甲基咪唑甲基硫酸盐和1-乙基-3-甲基咪唑硫氰酸盐与乙醇或1-丙醇的离子液体的四种二元混合物的密度,声速和粘度。整个合成范围内的(283.15–323.15)K范围内。从这些实验数据中,可以计算出纯组分和混合物的过量摩尔体积和等熵可压缩性,并使用Redlich-Kister多项式方程令人满意地拟合。可以证实,有机化合物对所研究的离子液体的热物理性质具有很强的影响。1-醇的烷基链长度的增加导致过量摩尔体积的过量偏差的增加。











































 京公网安备 11010802027423号
京公网安备 11010802027423号