当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Plutonium Redox Transformation in the Presence of Iron, Organic Matter, and Hydroxyl Radicals: Kinetics and Mechanistic Insights
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2021-01-20 , DOI: 10.1021/acs.est.0c08195 Chao Pan 1 , Yongqin Jiao 2 , Annie B. Kersting 1 , Mavrik Zavarin 1
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2021-01-20 , DOI: 10.1021/acs.est.0c08195 Chao Pan 1 , Yongqin Jiao 2 , Annie B. Kersting 1 , Mavrik Zavarin 1
Affiliation
|
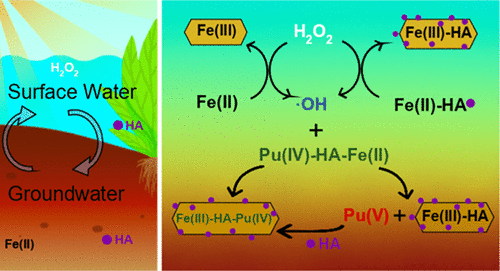
Plutonium (Pu) redox and complexation processes in the presence of natural organic matter and associated iron can impact the fate and transport of Pu in the environment. We studied the fate of Pu(IV) in the presence of humic acid (HA) and Fe(II) upon reaction with H2O2 that may be generated by photochemical and other reactions. A portion of Pu(IV) was oxidized to Pu(V/VI), which is primarily ascribed to the generation of reactive intermediates from the oxidation of Fe(II) and Fe(II)–HA complexes by H2O2. The kinetics of Pu(IV) oxidation is pH-dependent and can be described by a model that incorporates Pu redox kinetics with published HA-modified Fenton reaction kinetics. At pH 3.5, the presence of HA slowed Pu(IV) oxidation, while at pH 6, HA accelerated Pu(IV) oxidation in the first several hours followed by a reverse process where the oxidized Pu(V/VI) was reduced back to Pu(IV). Analysis of Pu-associated particle size suggests that Pu oxidation state is a major driver in its complexation with HA and formation of colloids and heteroaggregates. Our results revealed the H2O2-driven oxidation of Pu(IV)–HA–Fe(II) colloids with implications to the transient mobilization of Pu(V/VI) in organic-rich redox transition zones.
中文翻译:

铁,有机物和羟基自由基存在下Red的氧化还原转化:动力学和机理研究
在天然有机物和相关铁的存在下red(Pu)的氧化还原和络合过程会影响环境中Pu的命运和运输。我们研究了在腐殖酸(HA)和Fe(II)存在下与光化学反应和其他反应生成的H 2 O 2反应后Pu(IV)的命运。Pu(IV)的一部分被氧化为Pu(V / VI),这主要归因于H 2 O 2氧化Fe(II)和Fe(II)-HA配合物而生成的反应性中间体。Pu(IV)氧化的动力学是pH依赖性的,可以通过将Pu氧化还原动力学与已发表的HA修饰的Fenton反应动力学相结合的模型来描述。在pH 3.5时,HA的存在减慢了Pu(IV)的氧化,而在pH 6时,HA在头几个小时内加速了Pu(IV)的氧化,然后进行反向过程,其中氧化的Pu(V / VI)被还原为Pu(IV)。Pu相关粒径的分析表明,Pu的氧化态是其与HA形成络合物以及形成胶体和杂聚集体的主要驱动力。我们的结果表明,H 2 O 2驱动的Pu(IV)-HA-Fe(II)胶体氧化,对富含有机物的氧化还原过渡区中Pu(V / VI)的瞬时动员具有影响。
更新日期:2021-02-02
中文翻译:

铁,有机物和羟基自由基存在下Red的氧化还原转化:动力学和机理研究
在天然有机物和相关铁的存在下red(Pu)的氧化还原和络合过程会影响环境中Pu的命运和运输。我们研究了在腐殖酸(HA)和Fe(II)存在下与光化学反应和其他反应生成的H 2 O 2反应后Pu(IV)的命运。Pu(IV)的一部分被氧化为Pu(V / VI),这主要归因于H 2 O 2氧化Fe(II)和Fe(II)-HA配合物而生成的反应性中间体。Pu(IV)氧化的动力学是pH依赖性的,可以通过将Pu氧化还原动力学与已发表的HA修饰的Fenton反应动力学相结合的模型来描述。在pH 3.5时,HA的存在减慢了Pu(IV)的氧化,而在pH 6时,HA在头几个小时内加速了Pu(IV)的氧化,然后进行反向过程,其中氧化的Pu(V / VI)被还原为Pu(IV)。Pu相关粒径的分析表明,Pu的氧化态是其与HA形成络合物以及形成胶体和杂聚集体的主要驱动力。我们的结果表明,H 2 O 2驱动的Pu(IV)-HA-Fe(II)胶体氧化,对富含有机物的氧化还原过渡区中Pu(V / VI)的瞬时动员具有影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号