当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Visualization and Control of Chemically Induced Crack Formation in All-Solid-State Lithium-Metal Batteries with Sulfide Electrolyte
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2021-01-20 , DOI: 10.1021/acsami.0c18314 Misae Otoyama 1, 2 , Motoshi Suyama 1 , Chie Hotehama 1 , Hiroe Kowada 1 , Yoshihiro Takeda 3 , Koichiro Ito 3 , Atsushi Sakuda 1 , Masahiro Tatsumisago 1 , Akitoshi Hayashi 1
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2021-01-20 , DOI: 10.1021/acsami.0c18314 Misae Otoyama 1, 2 , Motoshi Suyama 1 , Chie Hotehama 1 , Hiroe Kowada 1 , Yoshihiro Takeda 3 , Koichiro Ito 3 , Atsushi Sakuda 1 , Masahiro Tatsumisago 1 , Akitoshi Hayashi 1
Affiliation
|
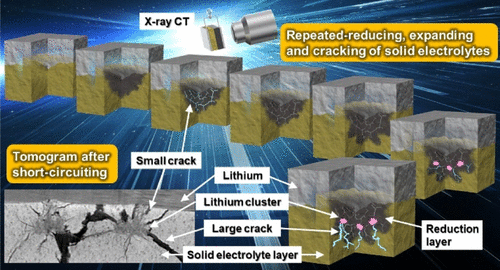
The application of lithium metal as a negative electrode in all-solid-state batteries shows promise for optimizing battery safety and energy density. However, further development relies on a detailed understanding of the chemo-mechanical issues at the interface between the lithium metal and solid electrolyte (SE). In this study, crack formation inside the sulfide SE (Li3PS4: LPS) layers during battery operation was visualized using in situ X-ray computed tomography (X-ray CT). Moreover, the degradation mechanism that causes short-circuiting was proposed based on a combination of the X-ray CT results and scanning electron microscopy images after short-circuiting. The primary cause of short-circuiting was a chemical reaction in which LPS was reduced at the lithium interface. The LPS expanded during decomposition, thereby forming small cracks. Lithium penetrated the small cracks to form new interfaces with fresh LPS on the interior of the LPS layers. This combination of reduction–expansion–cracking of LPS was repeated at these new interfaces. Lithium clusters eventually formed, thereby generating large cracks due to stress concentration. Lithium penetrated these large cracks easily, finally causing short-circuiting. Therefore, preventing the reduction reaction at the interface between the SE and lithium metal is effective in suppressing degradation. In fact, LPS-LiI electrolytes, which are highly stable to reduction, were demonstrated to prevent the repeated degradation mechanism. These findings will promote all-solid-state lithium-metal battery development by providing valuable insight into the design of the interface between SEs and lithium, where the selection of a suitable SE is vital.
中文翻译:

硫化电解质在全固态锂金属电池中化学诱导的裂纹形成的可视化和控制
金属锂作为负极在全固态电池中的应用显示出有望优化电池安全性和能量密度。然而,进一步的发展依赖于对锂金属和固体电解质(SE)之间界面化学化学问题的详细理解。在这项研究中,使用原位观察了电池操作过程中硫化物SE(Li 3 PS 4:LPS)层内部的裂纹形成X射线计算机断层扫描(X射线CT)。此外,基于X射线CT结果和短路后的扫描电子显微镜图像的组合,提出了引起短路的劣化机理。短路的主要原因是化学反应,其中锂界面处的LPS降低。LPS在分解过程中膨胀,从而形成小裂纹。锂穿透了小裂缝,在LPS层的内部与新鲜的LPS形成了新的界面。在这些新界面上,LPS的还原-膨胀-裂纹组合反复出现。最终形成锂团簇,从而由于应力集中而产生大的裂纹。锂很容易穿透这些大裂缝,最终导致短路。因此,防止SE和锂金属之间的界面处的还原反应对于抑制降解是有效的。实际上,已证明对还原高度稳定的LPS-LiI电解质可防止重复降解机理。这些发现将通过提供对SE和锂之间界面设计的宝贵见解,从而促进全固态锂金属电池的发展,而选择合适的SE至关重要。
更新日期:2021-02-03
中文翻译:

硫化电解质在全固态锂金属电池中化学诱导的裂纹形成的可视化和控制
金属锂作为负极在全固态电池中的应用显示出有望优化电池安全性和能量密度。然而,进一步的发展依赖于对锂金属和固体电解质(SE)之间界面化学化学问题的详细理解。在这项研究中,使用原位观察了电池操作过程中硫化物SE(Li 3 PS 4:LPS)层内部的裂纹形成X射线计算机断层扫描(X射线CT)。此外,基于X射线CT结果和短路后的扫描电子显微镜图像的组合,提出了引起短路的劣化机理。短路的主要原因是化学反应,其中锂界面处的LPS降低。LPS在分解过程中膨胀,从而形成小裂纹。锂穿透了小裂缝,在LPS层的内部与新鲜的LPS形成了新的界面。在这些新界面上,LPS的还原-膨胀-裂纹组合反复出现。最终形成锂团簇,从而由于应力集中而产生大的裂纹。锂很容易穿透这些大裂缝,最终导致短路。因此,防止SE和锂金属之间的界面处的还原反应对于抑制降解是有效的。实际上,已证明对还原高度稳定的LPS-LiI电解质可防止重复降解机理。这些发现将通过提供对SE和锂之间界面设计的宝贵见解,从而促进全固态锂金属电池的发展,而选择合适的SE至关重要。











































 京公网安备 11010802027423号
京公网安备 11010802027423号