当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Cascade Rh(III)‐catalyzed C−H Activation/Chemodivergent Annulation of N‐carbamoylindoles with Sulfoxonium Ylides for the Synthesis of Dihydropyrimidoindolone and Tricyclic [1,3]Oxazino[3,4‐a]indol‐1‐ones Derivatives
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2021-01-21 , DOI: 10.1002/adsc.202001380 Hui Xie 1 , Mei Zhong 2 , Hua‐Jie Kang 2 , Bing Shu 2 , Shang‐Shi Zhang 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2021-01-21 , DOI: 10.1002/adsc.202001380 Hui Xie 1 , Mei Zhong 2 , Hua‐Jie Kang 2 , Bing Shu 2 , Shang‐Shi Zhang 1
Affiliation
|
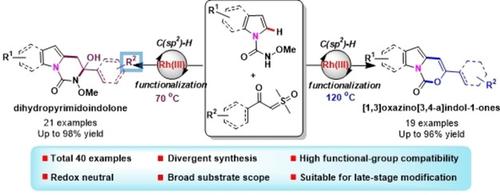
A highly efficient cascade Rh(III)‐catalyzed C−H activation/intramolecular chemodivergent cyclization reaction of N‐carbamoylindoles and sulfoxonium ylides has been successfully achieved for the first time. This synergistic process provides rapid access to functionalized dihydropyrimidoindolone and tricyclic [1,3]oxazino[3,4‐a]indol‐1‐ones skeletons under redox neutral conditions with broad substrate scope and remarkable functional‐group compatibility. Further late‐stage modification of structurally complex drug molecules and mechanistic studies were also accomplished.
中文翻译:

级联的Rh(III)催化N-氨基甲酰基吲哚与亚砜基内酯的CH-H活化/化学发散环化反应合成二氢嘧啶并吲哚酮和三环[1,3] Oxazino [3,4-a]吲哚-1-ones衍生物
首次成功实现了高效的级联Rh(III)催化的N-氨基甲酰基吲哚和亚砜基鎓盐的CH活化/分子内化学发散环化反应。此协同过程可在氧化还原中性条件下快速获得官能化的二氢嘧啶基吲哚酮和三环[1,3]恶嗪[3,4- a ]吲哚-1-酮骨架,具有广泛的底物范围和显着的官能团相容性。还完成了对结构复杂的药物分子的进一步后期修饰和机理研究。
更新日期:2021-03-03
中文翻译:

级联的Rh(III)催化N-氨基甲酰基吲哚与亚砜基内酯的CH-H活化/化学发散环化反应合成二氢嘧啶并吲哚酮和三环[1,3] Oxazino [3,4-a]吲哚-1-ones衍生物
首次成功实现了高效的级联Rh(III)催化的N-氨基甲酰基吲哚和亚砜基鎓盐的CH活化/分子内化学发散环化反应。此协同过程可在氧化还原中性条件下快速获得官能化的二氢嘧啶基吲哚酮和三环[1,3]恶嗪[3,4- a ]吲哚-1-酮骨架,具有广泛的底物范围和显着的官能团相容性。还完成了对结构复杂的药物分子的进一步后期修饰和机理研究。









































 京公网安备 11010802027423号
京公网安备 11010802027423号