当前位置:
X-MOL 学术
›
Genes Cells
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Di‐lysine motif‐like sequences formed by deleting the C‐terminal domain of aquaporin‐4 prevent its trafficking to the plasma membrane
Genes to Cells ( IF 1.3 ) Pub Date : 2021-01-21 , DOI: 10.1111/gtc.12829 Simon Chau 1 , Atsushi Fujii 1 , Yingqi Wang 1 , Arno Vandebroek 1 , Wakami Goda 1 , Masato Yasui 1, 2 , Yoichiro Abe 1, 2
Genes to Cells ( IF 1.3 ) Pub Date : 2021-01-21 , DOI: 10.1111/gtc.12829 Simon Chau 1 , Atsushi Fujii 1 , Yingqi Wang 1 , Arno Vandebroek 1 , Wakami Goda 1 , Masato Yasui 1, 2 , Yoichiro Abe 1, 2
Affiliation
|
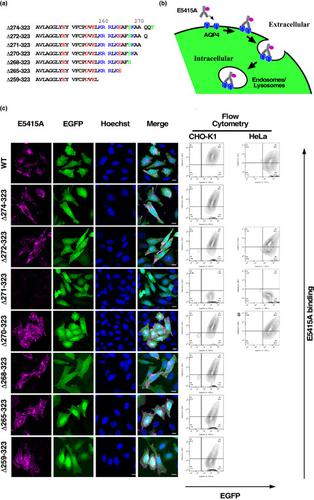
Aquaporin‐4 is a transmembrane water channel protein, the C‐terminal domain of which is facing the cytosol. In the process of investigating the role of the C‐terminal domain of aquaporin‐4 with regard to intracellular trafficking, we observed that a derivative of aquaporin‐4, in which the C‐terminal 53 amino acids had been removed (Δ271‐323), was localized to intracellular compartments, including the endoplasmic reticulum, but was not expressed on the plasma membranes. This was determined by immunofluorescence staining and labeling of the cells with monoclonal antibody specifically recognizing the extracellular domain of aquaporin‐4, followed by confocal microscopy and flow cytometry. Deletion of additional amino acids in the C‐terminal domain of aquaporin‐4 led to its redistribution to the plasma membrane. This suggests that the effect of the 53‐amino acid deletion on the subcellular localization of aquaporin‐4 could be attributed to the formation of a signal at the C terminus that retained aquaporin‐4 in intracellular compartments, rather than the loss of a signal required for plasma membrane targeting. Substitution of the lysine at position 268 with alanine could rescue the Δ271‐323‐associated retention in the cytosol, suggesting that the C‐terminal sequence of the mutant served as a signal similar to a di‐lysine motif.
中文翻译:

通过删除水通道蛋白4的C末端结构域形成的二赖氨酸基序样序列可防止其转运至质膜
Aquaporin-4是跨膜水通道蛋白,其C端结构域面对细胞质。在研究水通道蛋白4的C末端域在细胞内运输方面的作用的过程中,我们观察到了水通道蛋白4的衍生物,其中C末端的53个氨基酸被去除了(Δ271-323) ,定位于细胞内区室,包括内质网,但不表达在质膜上。这是通过免疫荧光染色和使用特异性识别水通道蛋白4细胞外结构域的单克隆抗体标记细胞,然后进行共聚焦显微镜和流式细胞术确定的。水通道蛋白4 C末端结构域中其他氨基酸的删除导致其重新分布到质膜上。这表明53个氨基酸的缺失对aquaporin-4亚细胞定位的影响可能是由于在C末端形成了信号,该信号在细胞内区室中保留了aquaporin-4,而不是丢失了所需的信号用于质膜靶向。赖氨酸在268位被丙氨酸取代可以挽救Δ271-323相关的胞质保留,这表明该突变体的C端序列起着类似于二赖氨酸基序的信号作用。
更新日期:2021-03-08
中文翻译:

通过删除水通道蛋白4的C末端结构域形成的二赖氨酸基序样序列可防止其转运至质膜
Aquaporin-4是跨膜水通道蛋白,其C端结构域面对细胞质。在研究水通道蛋白4的C末端域在细胞内运输方面的作用的过程中,我们观察到了水通道蛋白4的衍生物,其中C末端的53个氨基酸被去除了(Δ271-323) ,定位于细胞内区室,包括内质网,但不表达在质膜上。这是通过免疫荧光染色和使用特异性识别水通道蛋白4细胞外结构域的单克隆抗体标记细胞,然后进行共聚焦显微镜和流式细胞术确定的。水通道蛋白4 C末端结构域中其他氨基酸的删除导致其重新分布到质膜上。这表明53个氨基酸的缺失对aquaporin-4亚细胞定位的影响可能是由于在C末端形成了信号,该信号在细胞内区室中保留了aquaporin-4,而不是丢失了所需的信号用于质膜靶向。赖氨酸在268位被丙氨酸取代可以挽救Δ271-323相关的胞质保留,这表明该突变体的C端序列起着类似于二赖氨酸基序的信号作用。







































 京公网安备 11010802027423号
京公网安备 11010802027423号