Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hierarchical approach for the rational construction of helix-containing nanofibrils using α,β-peptides
Nanoscale ( IF 5.8 ) Pub Date : 2021-1-13 , DOI: 10.1039/d0nr04313c Monika Szefczyk 1 , Natalia Szulc 2 , Marlena Gąsior-Głogowska 2 , Anna Modrak-Wójcik 3 , Agnieszka Bzowska 3 , Wojciech Majstrzyk 4 , Michał Taube 5 , Maciej Kozak 5, 6 , Teodor Gotszalk 4 , Ewa Rudzińska-Szostak 1 , Łukasz Berlicki 1
Nanoscale ( IF 5.8 ) Pub Date : 2021-1-13 , DOI: 10.1039/d0nr04313c Monika Szefczyk 1 , Natalia Szulc 2 , Marlena Gąsior-Głogowska 2 , Anna Modrak-Wójcik 3 , Agnieszka Bzowska 3 , Wojciech Majstrzyk 4 , Michał Taube 5 , Maciej Kozak 5, 6 , Teodor Gotszalk 4 , Ewa Rudzińska-Szostak 1 , Łukasz Berlicki 1
Affiliation
|
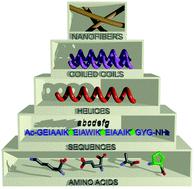
The rational design of novel self-assembled nanomaterials based on peptides remains a great challenge in modern chemistry. A hierarchical approach for the construction of nanofibrils based on α,β-peptide foldamers is proposed. The incorporation of a helix-promoting trans-(1S,2S)-2-aminocyclopentanecarboxylic acid residue in the outer positions of the model coiled-coil peptide led to its increased conformational stability, which was established consistently by the results of CD, NMR and FT-IR spectroscopy. The designed oligomerization state in the solution of the studied peptides was confirmed using analytical ultracentrifugation. Moreover, the cyclopentane side chain allowed additional interactions between coiled-coil-like structures to direct the self-assembly process towards the formation of well-defined nanofibrils, as observed using AFM and TEM techniques.
中文翻译:

使用α,β-肽合理构建含螺旋纳米纤维的分层方法
基于肽的新型自组装纳米材料的合理设计仍然是现代化学的一大挑战。提出了一种基于α,β-肽折叠体构建纳米纤丝的分层方法。加入促进螺旋的反式-(1 S ,2 S)-2-氨基环戊烷羧酸残基在模型卷曲螺旋肽的外部位置导致其构象稳定性增加,这与 CD、NMR 和 FT-IR 光谱的结果一致。使用分析超速离心法证实了所研究肽溶液中设计的寡聚状态。此外,如使用 AFM 和 TEM 技术所观察到的,环戊烷侧链允许盘绕线圈状结构之间的额外相互作用,以引导自组装过程形成明确定义的纳米原纤维。
更新日期:2021-01-20
中文翻译:

使用α,β-肽合理构建含螺旋纳米纤维的分层方法
基于肽的新型自组装纳米材料的合理设计仍然是现代化学的一大挑战。提出了一种基于α,β-肽折叠体构建纳米纤丝的分层方法。加入促进螺旋的反式-(1 S ,2 S)-2-氨基环戊烷羧酸残基在模型卷曲螺旋肽的外部位置导致其构象稳定性增加,这与 CD、NMR 和 FT-IR 光谱的结果一致。使用分析超速离心法证实了所研究肽溶液中设计的寡聚状态。此外,如使用 AFM 和 TEM 技术所观察到的,环戊烷侧链允许盘绕线圈状结构之间的额外相互作用,以引导自组装过程形成明确定义的纳米原纤维。









































 京公网安备 11010802027423号
京公网安备 11010802027423号