当前位置:
X-MOL 学术
›
J. Mater. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oral nanotherapeutics with enhanced mucus penetration and ROS-responsive drug release capacities for delivery of curcumin to colitis tissues
Journal of Materials Chemistry B ( IF 6.1 ) Pub Date : 2020-12-16 , DOI: 10.1039/d0tb02092c Yamei Huang 1, 2, 3, 4, 5 , Brandon S. B. Canup 6, 7, 8, 9 , Shuangquan Gou 4, 5, 10, 11, 12 , Nanxi Chen 1, 2, 3, 4, 5 , Fangyin Dai 1, 2, 3, 4, 5 , Bo Xiao 1, 2, 3, 4, 5 , Changming Li 4, 5, 10, 11, 12
Journal of Materials Chemistry B ( IF 6.1 ) Pub Date : 2020-12-16 , DOI: 10.1039/d0tb02092c Yamei Huang 1, 2, 3, 4, 5 , Brandon S. B. Canup 6, 7, 8, 9 , Shuangquan Gou 4, 5, 10, 11, 12 , Nanxi Chen 1, 2, 3, 4, 5 , Fangyin Dai 1, 2, 3, 4, 5 , Bo Xiao 1, 2, 3, 4, 5 , Changming Li 4, 5, 10, 11, 12
Affiliation
|
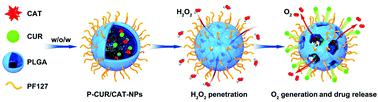
The therapeutic efficacies of oral nanotherapeutics for ulcerative colitis (UC) are seriously hindered by the lack of mucus-penetrating capacity and uncontrolled drug release. To overcome these limitations, the surface of poly(lactic-co-glycolic acid) (PLGA)-based nanoparticles (NPs) was functionalized with pluronic F127 (PF127), and catalase (CAT)/curcumin (CUR) was co-encapsulated into these NPs. The obtained P-CUR/CAT-NPs had a hydrodynamic particle size of approximately 274.1 nm, narrow size distribution, negative zeta potential (−14.0 mV), and smooth surface morphology. Moreover, the introduction of PF127 to the surface of NPs not only facilitated their mucus penetration, but also improved their cellular uptake efficiency by the target cells (macrophages). We further found that the encapsulation of CAT could remarkably increase the release rate of CUR from NPs in the presence of an H2O2-rich environment. Additionally, P-CUR/CAT-NPs showed the strongest capacity to suppress the secretion of the main pro-inflammatory cytokines, in comparison with their counterparts (CUR-NPs and P-CUR-NPs). Importantly, oral administration of P-CAT/CUR-NPs showed the best therapeutic outcomes than the other NPs. Collectively, these results clearly demonstrate that these mucus-penetrating NPs loaded with CAT and CUR can be exploited as an efficient nanotherapeutic for UC therapy.
中文翻译:

口服纳米治疗剂,具有增强的粘液渗透性和ROS响应药物释放能力,可将姜黄素递送至结肠炎组织
缺乏黏液穿透能力和药物释放不受控制,严重阻碍了口腔纳米治疗溃疡性结肠炎(UC)的治疗效果。为了克服这些限制,聚表面(乳酸-共-乙醇酸(PLGA)为基础的纳米颗粒(NPs)被普鲁尼克F127(PF127)功能化,过氧化氢酶(CAT)/姜黄素(CUR)被共包封到这些NP中。所得的P-CUR / CAT-NPs的流体动力学粒径约为274.1 nm,粒径分布窄,ζ电位为负(-14.0 mV),表面形态光滑。此外,将PF127引入NP的表面不仅促进了它们的粘液渗透,而且提高了靶细胞(巨噬细胞)对细胞的吸收效率。我们还发现,在H 2 O 2存在的情况下,CAT的封装可以显着提高NPs释放CUR的速率。丰富的环境。此外,与它们的对应物(CUR-NP和P-CUR-NP)相比,P-CUR / CAT-NPs显示出最强的抑制主要促炎细胞因子分泌的能力。重要的是,口服P-CAT / CUR-NP比其他NP具有最佳的治疗效果。总体而言,这些结果清楚地表明,这些载有CAT和CUR的可穿透粘液的NP可作为UC治疗的有效纳米疗法。
更新日期:2021-01-20
中文翻译:

口服纳米治疗剂,具有增强的粘液渗透性和ROS响应药物释放能力,可将姜黄素递送至结肠炎组织
缺乏黏液穿透能力和药物释放不受控制,严重阻碍了口腔纳米治疗溃疡性结肠炎(UC)的治疗效果。为了克服这些限制,聚表面(乳酸-共-乙醇酸(PLGA)为基础的纳米颗粒(NPs)被普鲁尼克F127(PF127)功能化,过氧化氢酶(CAT)/姜黄素(CUR)被共包封到这些NP中。所得的P-CUR / CAT-NPs的流体动力学粒径约为274.1 nm,粒径分布窄,ζ电位为负(-14.0 mV),表面形态光滑。此外,将PF127引入NP的表面不仅促进了它们的粘液渗透,而且提高了靶细胞(巨噬细胞)对细胞的吸收效率。我们还发现,在H 2 O 2存在的情况下,CAT的封装可以显着提高NPs释放CUR的速率。丰富的环境。此外,与它们的对应物(CUR-NP和P-CUR-NP)相比,P-CUR / CAT-NPs显示出最强的抑制主要促炎细胞因子分泌的能力。重要的是,口服P-CAT / CUR-NP比其他NP具有最佳的治疗效果。总体而言,这些结果清楚地表明,这些载有CAT和CUR的可穿透粘液的NP可作为UC治疗的有效纳米疗法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号