当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Self-discharge of magnesium–sulfur batteries leads to active material loss and poor shelf life
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2021-1-12 , DOI: 10.1039/d0ee01578d Hunter O. Ford 1, 2, 3, 4 , Emily S. Doyle 1, 2, 3, 4 , Peng He 1, 2, 3, 4 , William C. Boggess 2, 3, 4, 5 , Allen G. Oliver 2, 3, 4, 5 , Tianpin Wu 4, 6, 7, 8, 9 , George E. Sterbinsky 4, 6, 7, 8, 9 , Jennifer L. Schaefer 1, 2, 3, 4
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2021-1-12 , DOI: 10.1039/d0ee01578d Hunter O. Ford 1, 2, 3, 4 , Emily S. Doyle 1, 2, 3, 4 , Peng He 1, 2, 3, 4 , William C. Boggess 2, 3, 4, 5 , Allen G. Oliver 2, 3, 4, 5 , Tianpin Wu 4, 6, 7, 8, 9 , George E. Sterbinsky 4, 6, 7, 8, 9 , Jennifer L. Schaefer 1, 2, 3, 4
Affiliation
|
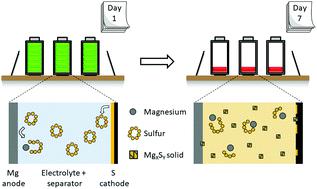
Due to its high theoretical energy density and relative abundancy of active materials, the magnesium–sulfur battery has attracted research attention in recent years. A closely related system, the lithium-sulfur battery, can suffer from serious self-discharge behavior. Until now, the self-discharge of Mg–S has been rarely addressed. Herein, we demonstrate for a wide variety of Mg–S electrolytes and conditions that Mg–S batteries also suffer from serious self-discharge. For a common Mg–S electrolyte, we identify a multi-step self-discharge pathway. Covalent S8 diffuses to the metal Mg anode and is converted to ionic Mg polysulfide in a non-faradaic reaction. Mg polysulfides in solution are found to be meta-stable, continuing to react and precipitate as solid magnesium polysulfide species during both storage and active use. Mg–S electrolytes from the early, middle, and state-of-the-art stages of the Mg–S literature are all found to enable the self-discharge. The self-discharge behavior is found to decrease first cycle discharge capacity by at least 32%, and in some cases up to 96%, indicating this is a phenomenon of the Mg–S chemistry that deserves focused attention.
中文翻译:

镁硫电池的自放电会导致活性物质损失和较差的保质期
镁硫电池由于其理论能量密度高和活性物质相对丰富,因此近年来引起了研究关注。密切相关的系统锂硫电池可能会遭受严重的自放电行为。到目前为止,很少解决Mg-S的自放电问题。在此,我们证明了Mg-S电解液的种类繁多以及Mg-S电池也遭受严重自放电的情况。对于常见的Mg-S电解质,我们确定了多步自放电途径。共价8扩散到金属镁阳极,并在非法拉第反应中转化为离子多硫化镁。发现溶液中的多硫化镁是亚稳定的,在储存和主动使用期间,都作为固态的多硫化镁物质继续反应和沉淀。Mg-S文献的早期,中期和最新阶段的Mg-S电解质均可实现自放电。发现自放电行为会使第一循环放电容量降低至少32%,在某些情况下会降低96%,这表明这种Mg-S化学现象值得引起关注。
更新日期:2021-01-20
中文翻译:

镁硫电池的自放电会导致活性物质损失和较差的保质期
镁硫电池由于其理论能量密度高和活性物质相对丰富,因此近年来引起了研究关注。密切相关的系统锂硫电池可能会遭受严重的自放电行为。到目前为止,很少解决Mg-S的自放电问题。在此,我们证明了Mg-S电解液的种类繁多以及Mg-S电池也遭受严重自放电的情况。对于常见的Mg-S电解质,我们确定了多步自放电途径。共价8扩散到金属镁阳极,并在非法拉第反应中转化为离子多硫化镁。发现溶液中的多硫化镁是亚稳定的,在储存和主动使用期间,都作为固态的多硫化镁物质继续反应和沉淀。Mg-S文献的早期,中期和最新阶段的Mg-S电解质均可实现自放电。发现自放电行为会使第一循环放电容量降低至少32%,在某些情况下会降低96%,这表明这种Mg-S化学现象值得引起关注。











































 京公网安备 11010802027423号
京公网安备 11010802027423号