当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Highly regioselective palladium-catalyzed domino reaction for post-functionalization of BODIPY
Chemical Communications ( IF 4.3 ) Pub Date : 2021-1-4 , DOI: 10.1039/d0cc08163a Sisi Wang 1, 2, 3, 4, 5 , Zhaoli Wang 1, 2, 3, 4, 5 , Hu Gao 1, 2, 3, 4, 5 , Liang Jiang 1, 2, 3, 4, 5 , Hui Liu 1, 2, 3, 4, 5 , Fan Wu 1, 2, 3, 4, 5 , Yue Zhao 1, 2, 3, 4, 5 , Kin Shing Chan 1, 2, 3, 4, 5 , Zhen Shen 1, 2, 3, 4, 5
Chemical Communications ( IF 4.3 ) Pub Date : 2021-1-4 , DOI: 10.1039/d0cc08163a Sisi Wang 1, 2, 3, 4, 5 , Zhaoli Wang 1, 2, 3, 4, 5 , Hu Gao 1, 2, 3, 4, 5 , Liang Jiang 1, 2, 3, 4, 5 , Hui Liu 1, 2, 3, 4, 5 , Fan Wu 1, 2, 3, 4, 5 , Yue Zhao 1, 2, 3, 4, 5 , Kin Shing Chan 1, 2, 3, 4, 5 , Zhen Shen 1, 2, 3, 4, 5
Affiliation
|
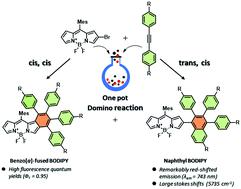
A series of benzo[a]-fused BODIPYs and the corresponding isomeric naphthyl-BODIPYs have been synthesized through a facile one-pot palladium-catalyzed domino reaction of BODIPY precursors (2-bromo-BODIPYs) with diarylethynes, via “cis, cis” and “trans, cis” addition transition states with alkynes, respectively. This reaction exhibits unprecedentedly complete cyclization regioselectivity to the a-position of the BODIPY over the b-one, and the resulting products can be effectively regulated by the substituent choice on the diarylethynes.
中文翻译:

高度区域选择性的钯催化的多米诺骨牌反应,用于BODIPY的后功能化
通过BODIPY前体(2-溴-BODIPYs)与二芳基乙炔的简单的一锅钯催化多米诺反应,通过“顺式,顺式”合成了一系列的苯并[ a ]稠合的BODIPY和相应的异构萘基-BODIPY。和“反式,顺式”分别与炔烃的加成过渡态。该反应对BODIPY的b位上的BODIPY的a位表现出前所未有的完全环化区域选择性,并且可以通过在二芳基乙炔上选择取代基来有效调节所得产物。
更新日期:2021-01-20
中文翻译:

高度区域选择性的钯催化的多米诺骨牌反应,用于BODIPY的后功能化
通过BODIPY前体(2-溴-BODIPYs)与二芳基乙炔的简单的一锅钯催化多米诺反应,通过“顺式,顺式”合成了一系列的苯并[ a ]稠合的BODIPY和相应的异构萘基-BODIPY。和“反式,顺式”分别与炔烃的加成过渡态。该反应对BODIPY的b位上的BODIPY的a位表现出前所未有的完全环化区域选择性,并且可以通过在二芳基乙炔上选择取代基来有效调节所得产物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号