当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ruthenium(II)-Catalyzed Direct C7-Selective Amidation of Indoles with Dioxazolones at Room Temperature
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-01-20 , DOI: 10.1021/acs.joc.0c02779 Yaoguang Sheng 1 , Jianmin Zhou 1 , Yi Gao 1 , Bingbing Duan 1 , Yi Wang 1 , Aleksandr Samorodov 2 , Guang Liang 1 , Qiuhua Zhao 3 , Zengqiang Song 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-01-20 , DOI: 10.1021/acs.joc.0c02779 Yaoguang Sheng 1 , Jianmin Zhou 1 , Yi Gao 1 , Bingbing Duan 1 , Yi Wang 1 , Aleksandr Samorodov 2 , Guang Liang 1 , Qiuhua Zhao 3 , Zengqiang Song 1
Affiliation
|
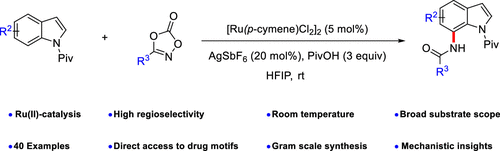
A protocol for the preparation of 7-amido indoles via regioselective C–H bond functionalization has been first accomplished under Ru(II) catalysis. Indole derivatives and 4-aryl/heteroaryl/benzyl/alkyl dioxzaolines containing various substituents were applicable for this transformation, readily providing the amidated indoles in moderate to good yields. This novel process has many advantages, including good compatibility with diverse functional groups, broad substrate scopes, and mild reaction conditions. Deuteration studies and control experiments have been performed to understand the mechanism of this transformation.
中文翻译:

室温下钌(II)催化吲哚与二恶唑酮的直接C7选择性酰胺化
通过Ru(II)催化首先完成了通过区域选择性C–H键官能化制备7-氨基吲哚的方案。含有各种取代基的吲哚衍生物和4-芳基/杂芳基/苄基/烷基二恶唑啉可用于该转化,容易地以中等至良好的产率提供酰胺化的吲哚。这种新颖的方法具有许多优点,包括与各种官能团的良好相容性,广泛的底物范围和温和的反应条件。已经进行了氘代研究和对照实验以了解这种转化的机理。
更新日期:2021-02-05
中文翻译:

室温下钌(II)催化吲哚与二恶唑酮的直接C7选择性酰胺化
通过Ru(II)催化首先完成了通过区域选择性C–H键官能化制备7-氨基吲哚的方案。含有各种取代基的吲哚衍生物和4-芳基/杂芳基/苄基/烷基二恶唑啉可用于该转化,容易地以中等至良好的产率提供酰胺化的吲哚。这种新颖的方法具有许多优点,包括与各种官能团的良好相容性,广泛的底物范围和温和的反应条件。已经进行了氘代研究和对照实验以了解这种转化的机理。











































 京公网安备 11010802027423号
京公网安备 11010802027423号