当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Direct Access for the Regio- and Stereoselective Synthesis of N-Alkenylpyrazoles and Chromenopyrazoles
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-01-19 , DOI: 10.1021/acs.joc.0c02421 Siva Hariprasad Kurma 1 , Balasubramanian Sridhar , China Raju Bhimapaka 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-01-19 , DOI: 10.1021/acs.joc.0c02421 Siva Hariprasad Kurma 1 , Balasubramanian Sridhar , China Raju Bhimapaka 1
Affiliation
|
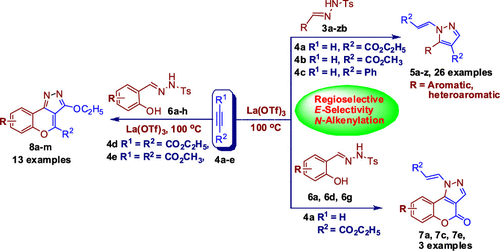
A highly regio- and stereoselective method was developed for the preparation of N-alkenylpyrazoles and chromenopyrazoles by the reaction of N-tosylhydrazones and salicyl N-tosylhydrazones with alkynes under neat conditions in the presence of La(OTf)3. The present study was found to be efficient and convenient for direct access to N-alkenylpyrazoles and chromenopyrazoles through C–C, C–N, and C–O bond forming reactions. Structure assignment of N-alkenylpyrazole compound 5c was confirmed by X-ray analysis.
中文翻译:

N-烯基吡唑和Chromenopyrazole的区域和立体选择性合成的直接途径
开发了一种高度区域选择性和立体选择性的方法,通过在纯净条件下,在La(OTf)3存在下,N-甲苯磺酰and和水杨基N-甲苯磺酰with与炔烃反应,制备N-烯基吡唑和苯并吡唑。发现本研究对于通过C–C,C–N和C–O键形成反应直接接触N-烯基吡唑和色吡唑是有效而便捷的。通过X射线分析确认了N-烯基吡唑化合物5c的结构归属。
更新日期:2021-02-05
中文翻译:

N-烯基吡唑和Chromenopyrazole的区域和立体选择性合成的直接途径
开发了一种高度区域选择性和立体选择性的方法,通过在纯净条件下,在La(OTf)3存在下,N-甲苯磺酰and和水杨基N-甲苯磺酰with与炔烃反应,制备N-烯基吡唑和苯并吡唑。发现本研究对于通过C–C,C–N和C–O键形成反应直接接触N-烯基吡唑和色吡唑是有效而便捷的。通过X射线分析确认了N-烯基吡唑化合物5c的结构归属。











































 京公网安备 11010802027423号
京公网安备 11010802027423号