当前位置:
X-MOL 学术
›
J. Phys. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Computational investigation of KICl2 iodination of thiophene and its electron‐poor derivatives
Journal of Physical Organic Chemistry ( IF 1.9 ) Pub Date : 2021-01-19 , DOI: 10.1002/poc.4190 Akash Mamon Sarkar 1 , Grigoriy A. Sereda 1 , Pere Miró 1
Journal of Physical Organic Chemistry ( IF 1.9 ) Pub Date : 2021-01-19 , DOI: 10.1002/poc.4190 Akash Mamon Sarkar 1 , Grigoriy A. Sereda 1 , Pere Miró 1
Affiliation
|
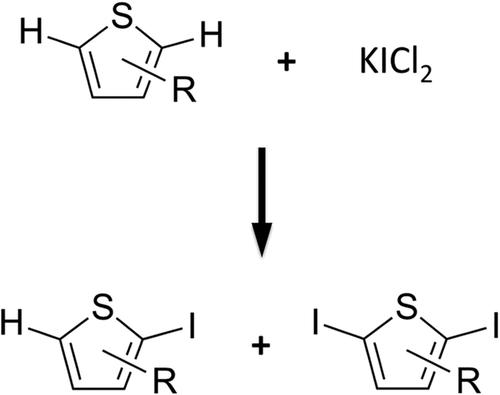
Electrophilic iodination of thiophene and its electron‐poor derivatives has been studied with KICl2 in dichloromethane and methanol using density function theory. KICl2 easily dissociates forming KCl and ICl, the latter being the iodinating agent. ICl forms an adduct with thiophene (π‐complex) followed by a nucleophilic attack of the ICl component by the aromatic component that forms the C–I bond. The nucleophilic attack is always the rate determining step with higher barriers that are in agreement the experimental conditions required for this reaction. The abstraction of a proton by the chloride anion on the last step is barrierless leading to the mono‐ and di‐iodination of the thiophene derivatives. Side products derived from the nucleophilic addition of the chloride anion have been determined to be transient.
中文翻译:

噻吩及其贫电子衍生物的KICl2碘化的计算研究
利用密度泛函理论,用KICl 2在二氯甲烷和甲醇中研究了噻吩及其贫电子衍生物的亲电碘化反应。氯化钾2容易离解形成KCl和ICl,后者为碘化剂。ICl与噻吩(π络合物)形成加合物,随后ICl组分被形成C–I键的芳族化合物发生亲核攻击。亲核攻击始终是具有较高壁垒的速率确定步骤,该壁垒与该反应所需的实验条件一致。在最后一步,氯阴离子提取质子是无障碍的,从而导致噻吩衍生物的单碘和二碘化。已确定衍生自氯化物阴离子亲核加成的副产物是瞬时的。
更新日期:2021-01-19
中文翻译:

噻吩及其贫电子衍生物的KICl2碘化的计算研究
利用密度泛函理论,用KICl 2在二氯甲烷和甲醇中研究了噻吩及其贫电子衍生物的亲电碘化反应。氯化钾2容易离解形成KCl和ICl,后者为碘化剂。ICl与噻吩(π络合物)形成加合物,随后ICl组分被形成C–I键的芳族化合物发生亲核攻击。亲核攻击始终是具有较高壁垒的速率确定步骤,该壁垒与该反应所需的实验条件一致。在最后一步,氯阴离子提取质子是无障碍的,从而导致噻吩衍生物的单碘和二碘化。已确定衍生自氯化物阴离子亲核加成的副产物是瞬时的。







































 京公网安备 11010802027423号
京公网安备 11010802027423号