当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Construction of N‐Boc‐2‐Alkylaminoquinazolin‐4(3H)‐Ones via a Three‐Component, One‐Pot Protocol Mediated by Copper(II) Chloride that Spares Enantiomeric Purity
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2021-01-19 , DOI: 10.1002/adsc.202001279 Xiaoyu Li 1 , Jennifer E Golden 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2021-01-19 , DOI: 10.1002/adsc.202001279 Xiaoyu Li 1 , Jennifer E Golden 1
Affiliation
|
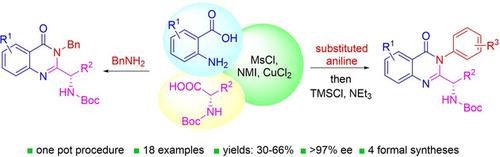
Chiral 2‐alkylquinazolinones are key synthetic intermediates, but their preparation in high optical purity is challenging. Thus, a multicomponent procedure integrating anthranilic acids, N‐Boc‐amino acids, and amines in the presence of methanesulfonyl chloride, N‐methylimidazole, and copper(II) chloride was developed to mildly afford N‐Boc‐2‐alkylaminoquinazolin‐4(3H)‐ones with excellent preservation of enantiomeric purity (>99% ee). Copper(II) chloride was essential to retaining enantiopurity, and reaction component structural changes were well tolerated, resulting in an efficient, all‐in‐one procedure that promotes sequential coupling, lactonization, aminolysis, and cyclization in good yields. The method was applied to the rapid assembly of four key intermediates used in the synthesis of high profile quinazolinones, including several PI3K inhibitor drugs.
中文翻译:

通过氯化铜(II)介导的三组分一锅法构建 N-Boc-2-烷基氨基喹唑啉-4(3H)-Ones,保证对映体纯度
手性2-烷基喹唑啉酮是关键的合成中间体,但其高光学纯度的制备具有挑战性。因此,开发了一种在甲磺酰氯、 N-甲基咪唑和氯化铜(II)存在下整合邻氨基苯甲酸、 N -Boc-氨基酸和胺的多组分程序,以温和地提供N -Boc-2-烷基氨基喹唑啉-4( 3 H )-具有出色的对映体纯度保留 (>99% ee)。氯化铜(II)对于保持对映体纯度至关重要,并且反应组分结构变化具有良好的耐受性,从而形成一种高效的一体化程序,促进连续偶联、内酯化、氨解和环化,并获得良好的收率。该方法适用于快速组装用于合成高浓度喹唑啉酮类药物的四种关键中间体,包括几种 PI3K 抑制剂药物。
更新日期:2021-03-16
中文翻译:

通过氯化铜(II)介导的三组分一锅法构建 N-Boc-2-烷基氨基喹唑啉-4(3H)-Ones,保证对映体纯度
手性2-烷基喹唑啉酮是关键的合成中间体,但其高光学纯度的制备具有挑战性。因此,开发了一种在甲磺酰氯、 N-甲基咪唑和氯化铜(II)存在下整合邻氨基苯甲酸、 N -Boc-氨基酸和胺的多组分程序,以温和地提供N -Boc-2-烷基氨基喹唑啉-4( 3 H )-具有出色的对映体纯度保留 (>99% ee)。氯化铜(II)对于保持对映体纯度至关重要,并且反应组分结构变化具有良好的耐受性,从而形成一种高效的一体化程序,促进连续偶联、内酯化、氨解和环化,并获得良好的收率。该方法适用于快速组装用于合成高浓度喹唑啉酮类药物的四种关键中间体,包括几种 PI3K 抑制剂药物。









































 京公网安备 11010802027423号
京公网安备 11010802027423号