当前位置:
X-MOL 学术
›
J. Chin. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stille coupling for the synthesis of isoflavones by a reusable palladium catalyst in water
Journal of the Chinese Chemical Society ( IF 1.6 ) Pub Date : 2021-01-20 , DOI: 10.1002/jccs.202000478 Ya‐Ting Chang, Ling‐Jun Liu, Wen‐Sheng Peng, Lin‐Ting Lin, Yi‐Tsu Chan, Fu‐Yu Tsai
Journal of the Chinese Chemical Society ( IF 1.6 ) Pub Date : 2021-01-20 , DOI: 10.1002/jccs.202000478 Ya‐Ting Chang, Ling‐Jun Liu, Wen‐Sheng Peng, Lin‐Ting Lin, Yi‐Tsu Chan, Fu‐Yu Tsai
|
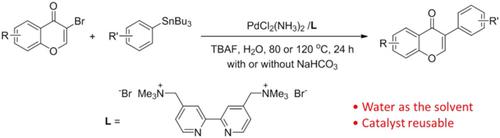
Isoflavones were synthesized from the reaction of 3‐bromochromone derivatives and aryltributylstannanes via Stille coupling catalyzed by a water‐soluble and reusable PdCl2(NH3)2/2,2′‐cationic bipyridyl system in aqueous solution. For prototype 3‐bromochromone, the coupling reaction was performed at 80°C for 24 hr with 2.5 mol% catalyst in water in the presence of tetrabutylammonium fluoride. After the reaction, the aqueous solution could be reused for several runs, indicating that its activity was only slightly decreased. For substituted 3‐bromochromones, the addition of NaHCO3 and a higher reaction temperature (120°C) were required to gain satisfactory outcomes. In addition, naturally occurring products, such as daidzein, could be obtained by this protocol via a one‐pot reaction.
中文翻译:

在水中可重复使用的钯催化剂用于合成异黄酮的Stille偶联
异黄酮是通过水溶性可重用的PdCl 2(NH 3)2 / 2,2'-阳离子联吡啶系统在水溶液中催化的Stille偶联反应,由3-溴色酮衍生物与芳基三丁基锡烷反应制得的。对于原型3-溴苯并二氢呋喃酮,偶合反应是在氟化四丁基铵存在下,在水中使用2.5 mol%催化剂,在80°C下进行24小时。反应后,该水溶液可重复使用几次,表明其活性仅略有下降。对于取代的3-溴色酮,添加NaHCO 3需要较高的反应温度(120°C)才能获得满意的结果。此外,该方案可通过一锅反应获得天然产物,如黄豆苷元。
更新日期:2021-03-26
中文翻译:

在水中可重复使用的钯催化剂用于合成异黄酮的Stille偶联
异黄酮是通过水溶性可重用的PdCl 2(NH 3)2 / 2,2'-阳离子联吡啶系统在水溶液中催化的Stille偶联反应,由3-溴色酮衍生物与芳基三丁基锡烷反应制得的。对于原型3-溴苯并二氢呋喃酮,偶合反应是在氟化四丁基铵存在下,在水中使用2.5 mol%催化剂,在80°C下进行24小时。反应后,该水溶液可重复使用几次,表明其活性仅略有下降。对于取代的3-溴色酮,添加NaHCO 3需要较高的反应温度(120°C)才能获得满意的结果。此外,该方案可通过一锅反应获得天然产物,如黄豆苷元。











































 京公网安备 11010802027423号
京公网安备 11010802027423号