Cellular and Molecular Gastroenterology and Hepatology ( IF 7.1 ) Pub Date : 2021-01-20 , DOI: 10.1016/j.jcmgh.2021.01.008 Rémi Samain 1 , Alexia Brunel 1 , Thibault Douché 1 , Marjorie Fanjul 1 , Stéphanie Cassant-Sourdy 1 , Julia Rochotte 1 , Jérôme Cros 2 , Cindy Neuzillet 3 , Jérôme Raffenne 1 , Camille Duluc 1 , Aurélie Perraud 4 , Jérémy Nigri 5 , Véronique Gigoux 1 , Ivan Bieche 6 , Matteo Ponzo 7 , Gilles Carpentier 7 , Ilaria Cascone 7 , Richard Tomasini 5 , Herbert A Schmid 8 , Muriel Mathonnet 4 , Rémy Nicolle 9 , Marie-Pierre Bousquet 10 , Yvan Martineau 1 , Stéphane Pyronnet 1 , Christine Jean 1 , Corinne Bousquet 1
|
Background & Aims
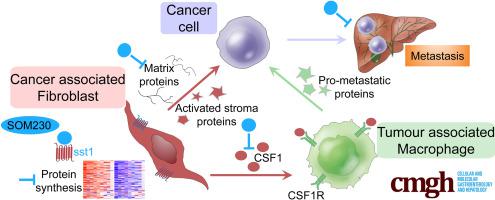
Cancer-associated fibroblasts (CAFs) from pancreatic adenocarcinoma (PDA) present high protein synthesis rates. CAFs express the G-protein–coupled somatostatin receptor sst1. The sst1 agonist SOM230 blocks CAF protumoral features in vitro and in immunocompromised mice. We have explored here the therapeutic potential of SOM230, and underlying mechanisms, in immunocompetent models of murine PDA mimicking the heavy fibrotic and immunosuppressive stroma observed in patient tumors.
Methods
Large-scale mass spectrometry analyses were performed on media conditioned from 9 patient PDA-derived CAF primary cultures. Spontaneous transgenic and experimental (orthotopic co-graft of tumor cells plus CAFs) PDA-bearing mice were longitudinally ultrasound-monitored for tumor and metastatic progression. Histopathology and flow cytometry analyses were performed on primary tumors and metastases. Stromal signatures were functionally validated through bioinformatics using several published, and 1 original, PDA database.
Results
Proteomics on the CAF secretome showed that SOM230 controls stromal activities including inflammatory responses. Among the identified secreted proteins, we validated that colony-stimulating factor 1 (CSF-1) (a macrophage growth factor) was reduced by SOM230 in the tumor and plasma of PDA-harboring mice, alongside intratumor stromal normalization (reduced CAF and macrophage activities), and dramatic metastasis reduction. In transgenic mice, these SOM230 benefits alleviate the chemotherapy-induced (gemcitabine) immunosuppressive stroma reshaping. Mechanistically, SOM230 acts in vivo on CAFs through sst1 to disrupt prometastatic CAF production of CSF-1 and cross-talk with macrophages. We found that in patients, stromal CSF-1 was associated with aggressive PDA forms.
Conclusions
We propose SOM230 as an antimetastatic therapy in PDA for its capacity to remodel the fibrotic and immunosuppressive myeloid stroma. This pharmacotherapy should benefit PDA patients treated with chemotherapies.
中文翻译:

胰腺癌相关成纤维细胞分泌组的药理学正常化损害与巨噬细胞的前转移交叉对话
背景与目标
来自胰腺腺癌 (PDA) 的癌症相关成纤维细胞 (CAF) 具有高蛋白质合成率。CAF 表达 G 蛋白偶联的生长抑素受体 sst1。sst1 激动剂 SOM230 在体外和免疫功能低下的小鼠中阻断 CAF 前肿瘤特征。我们在此探索了 SOM230 的治疗潜力和潜在机制,在小鼠 PDA 的免疫活性模型中模拟了在患者肿瘤中观察到的重度纤维化和免疫抑制基质。
方法
对来自 9 名患者 PDA 衍生的 CAF 原代培养物的培养基进行了大规模质谱分析。自发转基因和实验性(肿瘤细胞加 CAF 的原位共移植)携带 PDA 的小鼠被纵向超声监测肿瘤和转移进展。对原发性肿瘤和转移灶进行组织病理学和流式细胞术分析。基质特征通过生物信息学使用几个已发布的和 1 个原始 PDA 数据库进行功能验证。
结果
CAF 分泌组的蛋白质组学显示 SOM230 控制包括炎症反应在内的基质活动。在已鉴定的分泌蛋白中,我们验证了 SOM230 在携带 PDA 的小鼠的肿瘤和血浆中降低了集落刺激因子 1 (CSF-1)(一种巨噬细胞生长因子),同时肿瘤内基质正常化(CAF 和巨噬细胞活性降低) ),并显着减少转移。在转基因小鼠中,这些 SOM230 的益处减轻了化疗诱导的(吉西他滨)免疫抑制基质重塑。从机制上讲,SOM230 通过 sst1 在体内作用于 CAF,以破坏 CSF-1 的前转移性 CAF 产生和与巨噬细胞的串扰。我们发现在患者中,基质 CSF-1 与侵袭性 PDA 形式相关。
结论
我们建议将 SOM230 作为 PDA 的抗转移疗法,因为它具有重塑纤维化和免疫抑制骨髓基质的能力。这种药物疗法应该有益于接受化学疗法治疗的 PDA 患者。











































 京公网安备 11010802027423号
京公网安备 11010802027423号