Reactive & Functional Polymers ( IF 4.5 ) Pub Date : 2021-01-20 , DOI: 10.1016/j.reactfunctpolym.2021.104823 Alexander V. Pastukhov
|
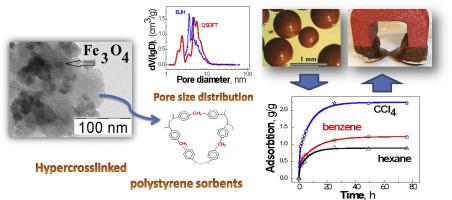
Magnetic sorbents were obtained by the immobilization of iron oxides into the pores of hypercrosslinked polystyrene sorbents via chemical precipitation. Various types of industrial Macronet and Amberlite adsorbents and the synthesized hypercrosslinked styrene-divinylbenzene copolymer were used as a porous polymer matrix. The volume fraction of the inorganic phase in the composites was 2.2–8.3 vol%. According to X-ray phase analysis and transmission electron microscopy, the inorganic phase in the sorbents was magnetite and the size of magnetite nanocrystallites was 3–16 nm. The structural studies of the composite sorbents by scanning electron microscopy/energy-dispersive X-ray spectrometry demonstrated a uniform distribution of nanodispersed iron oxides over the volume of sorbent granules. Investigation of the porous structure of magnetic sorbents by the low-temperature nitrogen sorption showed that the composites have a well-developed system of pores with a specific pore area of up to 1400 m2 g−1 and the volume of micropores (<3 nm) up to 0.6 cm3 g−1. The sorbent porosity parameters were calculated using methods based on the capillary condensation theory and the quenched solid density functional theory. Analysis of pore size distributions revealed that several specific pore “fractions” are present in the porous system of the magnetic sorbents. Micropores up to 2.5 nm in size were characteristic of all the studied sorbents; for some sorbent types, two to three additional “fractions” of mesopores up to 10 nm in size and macropores with sizes above 50 nm were detected. In the magnetic sorbents, the total pore volume was 0.4–1.0 cm3 g−1 and the mesopore volume was 0.2–0.6 cm3 g−1. It was found that the magnetic sorbents can adsorb toxic organic compounds of various classes: alcohols, ethers, and aliphatic, aromatic, and chlorine-containing hydrocarbons (from 0.4 to 1.0 mL cm−3). The obtained composite sorbents show promise for the purification of liquid, gaseous, and solid media by the magnetic separation method.
中文翻译:

基于苯乙烯和二乙烯基苯与固定化铁氧化物的超交联共聚物的磁性吸附剂
磁性吸附剂是通过化学沉淀将氧化铁固定在超交联聚苯乙烯吸附剂的孔中而获得的。各种类型的工业Macronet和Amberlite吸附剂以及合成的超交联苯乙烯-二乙烯基苯共聚物均用作多孔聚合物基质。复合材料中无机相的体积分数为2.2–8.3 vol%。根据X射线相分析和透射电子显微镜,吸附剂中的无机相为磁铁矿,磁铁矿纳米晶体的尺寸为3–16 nm。通过扫描电子显微镜/能量分散X射线光谱法对复合吸附剂的结构研究表明,纳米分散的氧化铁在吸附剂颗粒的体积上均匀分布。2 g -1和微孔的体积(<3 nm)可达0.6 cm 3 g -1。使用基于毛细管冷凝理论和淬灭固体密度泛函理论的方法计算吸附剂孔隙率参数。孔径分布的分析表明,磁性吸附剂的多孔系统中存在几个特定的孔“馏分”。所研究的所有吸附剂均具有尺寸最大为2.5 nm的微孔;对于某些类型的吸附剂,还可以检测到最多2到10 nm的中孔和另外50到50 nm以上的大孔的“三部分”。在磁性吸附剂中,总孔体积为0.4–1.0 cm 3 g -1,中孔体积为0.2–0.6 cm3 g -1。已经发现,磁性吸附剂可以吸附各种类型的有毒有机化合物:醇,醚以及脂族,芳族和含氯的烃(0.4至1.0mL cm -3)。所获得的复合吸附剂有望通过磁分离方法纯化液体,气体和固体介质。











































 京公网安备 11010802027423号
京公网安备 11010802027423号