当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
meso-borneol- and meso-carbazole-substituted porphyrins: multifunctional chromophores with tunable electronic structures and antitumor activities
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2021-1-1 , DOI: 10.1039/d0nj02954h Bo Fu 1, 2, 3, 4, 5 , Xinyi Dong 6, 7, 8, 9 , Xiaoxiao Yu 1, 2, 3, 4, 5 , Zhen Zhang 6, 7, 8, 9 , Lei Sun 1, 2, 3, 4, 5 , Weihua Zhu 6, 7, 8, 9 , Xu Liang 6, 7, 8, 9 , Haijun Xu 1, 2, 3, 4, 5
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2021-1-1 , DOI: 10.1039/d0nj02954h Bo Fu 1, 2, 3, 4, 5 , Xinyi Dong 6, 7, 8, 9 , Xiaoxiao Yu 1, 2, 3, 4, 5 , Zhen Zhang 6, 7, 8, 9 , Lei Sun 1, 2, 3, 4, 5 , Weihua Zhu 6, 7, 8, 9 , Xu Liang 6, 7, 8, 9 , Haijun Xu 1, 2, 3, 4, 5
Affiliation
|
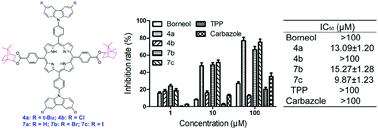
Herein, a series of five C2 symmetric H2porphyrins with meso-borneol and meso-carbazole units have been synthesized and isolated. An analysis of the electronic structures was carried out by spectroscopic investigations and electrochemical illustrations. In addition, the chiral optical properties observed in the CD spectra can be assigned to the interchromophore through-space coupling between the borneol chiral center and the porphyrinic chromophore. Further investigation of the anticancer behaviors shows that the modulation of the biological activity behaviors could be facilely achieved by introducing various halogen atoms on the carbazole rings.
中文翻译:

中冰片和中咔唑取代的卟啉:具有可调电子结构和抗肿瘤活性的多功能发色团
此处,一系列五个Ç 2对称ħ 2卟啉与内消旋-borneol和内消旋-咔唑单元已被合成并分离。通过光谱学研究和电化学图示对电子结构进行分析。此外,在CD光谱中观察到的手性光学性质可归因于冰片手性中心与卟啉发色团之间的发色团间空间耦合。对抗癌行为的进一步研究表明,可以通过在咔唑环上引入各种卤素原子来轻松实现生物活性行为的调节。
更新日期:2021-01-19
中文翻译:

中冰片和中咔唑取代的卟啉:具有可调电子结构和抗肿瘤活性的多功能发色团
此处,一系列五个Ç 2对称ħ 2卟啉与内消旋-borneol和内消旋-咔唑单元已被合成并分离。通过光谱学研究和电化学图示对电子结构进行分析。此外,在CD光谱中观察到的手性光学性质可归因于冰片手性中心与卟啉发色团之间的发色团间空间耦合。对抗癌行为的进一步研究表明,可以通过在咔唑环上引入各种卤素原子来轻松实现生物活性行为的调节。











































 京公网安备 11010802027423号
京公网安备 11010802027423号