当前位置:
X-MOL 学术
›
Environ. Sci.: Water Res. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrochemical degradation of perfluorinated compounds by Ag coated Ti (Ti/Ag) anode: electrode preparation, characterization and application
Environmental Science: Water Research & Technology ( IF 3.5 ) Pub Date : 2020-12-12 , DOI: 10.1039/d0ew00785d Jiawei Tang 1, 2, 3, 4 , Zong Liu 1, 2, 3, 4 , Wenjing Lu 1, 2, 3, 4 , Liangliang Wang 1, 2, 3, 4, 5 , Chunhui Zhang 1, 2, 3, 4 , Peidong Su 4, 6, 7, 8
Environmental Science: Water Research & Technology ( IF 3.5 ) Pub Date : 2020-12-12 , DOI: 10.1039/d0ew00785d Jiawei Tang 1, 2, 3, 4 , Zong Liu 1, 2, 3, 4 , Wenjing Lu 1, 2, 3, 4 , Liangliang Wang 1, 2, 3, 4, 5 , Chunhui Zhang 1, 2, 3, 4 , Peidong Su 4, 6, 7, 8
Affiliation
|
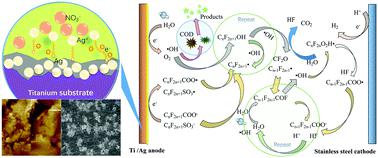
Perfluorinated compounds (PFCs) are environmentally persistent, bioaccumulative, and globally distributed pollutants, which exhibit potential toxicity to both humans and ecosystems. In this study, the electrochemical degradation of PFCs in the effluent of a municipal wastewater treatment plant (MWWTP) was conducted using an Ag coated titanium (Ti/Ag) anode. Results from the Tafel polarization analysis showed that the Ag film was uniformly coated on the surface of a Ti plate, and a higher corrosion potential (−0.3086 V) was obtained when compared with that of the original Ti electrode (−0.9707 V). To optimize the degradation process, response surface methodology (RSM) combined with a Box–Behnken design (BBD) was applied to optimize the factors that could affect the degradation of the PFCs. Results indicated that the maximum removal efficiencies of short chain PFCs (C–F < 7), long chain PFCs (C–F ≥ 7), and chemical oxygen demand (COD) were about 70.8%, 91.5%, and 92.0%, respectively, under optimum conditions, at which the current density was 20.0 mA cm−2, the pH was 6, the electrode distance was 1.6 cm within 100 min of electrolysis time. Moreover, triplicate tests were carried out and demonstrated that the relative standard deviation (RSD%) was lower than 5.0% which meant that the experimental design and the optimized factors were significant. The degradation kinetic analysis suggested that the degradation of COD and PFCs was in agreement with the pseudo-first-order kinetic reaction, and the COD degradation occurred prior to the long-chain PFCs and short-chain PFCs degradation.
中文翻译:

涂银的钛(Ti / Ag)阳极对全氟化合物的电化学降解:电极的制备,表征和应用
全氟化合物(PFC)是环境持久性,生物蓄积性和全球分布的污染物,对人类和生态系统均具有潜在毒性。在这项研究中,使用涂有Ag的钛(Ti / Ag)阳极进行了市政废水处理厂(MWWTP)废水中PFC的电化学降解。Tafel极化分析的结果表明,Ag膜均匀地涂覆在Ti板的表面上,与原始Ti电极的腐蚀电位(-0.9707 V)相比,获得了更高的腐蚀电位(-0.3086 V)。为了优化降解过程,应用了响应面方法(RSM)和Box-Behnken设计(BBD)来优化可能影响PFC降解的因素。-2,pH为6,在电解时间100分钟内电极距离为1.6厘米。此外,进行了三次测试,结果表明相对标准偏差(RSD%)低于5.0%,这意味着实验设计和优化因素非常重要。降解动力学分析表明,COD和PFC的降解与拟一级动力学反应一致,且COD的降解发生在长链PFC和短链PFC的降解之前。
更新日期:2021-01-19
中文翻译:

涂银的钛(Ti / Ag)阳极对全氟化合物的电化学降解:电极的制备,表征和应用
全氟化合物(PFC)是环境持久性,生物蓄积性和全球分布的污染物,对人类和生态系统均具有潜在毒性。在这项研究中,使用涂有Ag的钛(Ti / Ag)阳极进行了市政废水处理厂(MWWTP)废水中PFC的电化学降解。Tafel极化分析的结果表明,Ag膜均匀地涂覆在Ti板的表面上,与原始Ti电极的腐蚀电位(-0.9707 V)相比,获得了更高的腐蚀电位(-0.3086 V)。为了优化降解过程,应用了响应面方法(RSM)和Box-Behnken设计(BBD)来优化可能影响PFC降解的因素。-2,pH为6,在电解时间100分钟内电极距离为1.6厘米。此外,进行了三次测试,结果表明相对标准偏差(RSD%)低于5.0%,这意味着实验设计和优化因素非常重要。降解动力学分析表明,COD和PFC的降解与拟一级动力学反应一致,且COD的降解发生在长链PFC和短链PFC的降解之前。











































 京公网安备 11010802027423号
京公网安备 11010802027423号