当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Brönsted Basicities and Nucleophilicities of N-Heterocyclic Olefins in Solution: N-Heterocyclic Carbene versus N-Heterocyclic Olefin. Which Is More Basic, and Which Is More Nucleophilic?
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-01-19 , DOI: 10.1021/acs.joc.0c02838 Zhen Li 1 , Pengju Ji 1 , Jin-Pei Cheng 1, 2
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-01-19 , DOI: 10.1021/acs.joc.0c02838 Zhen Li 1 , Pengju Ji 1 , Jin-Pei Cheng 1, 2
Affiliation
|
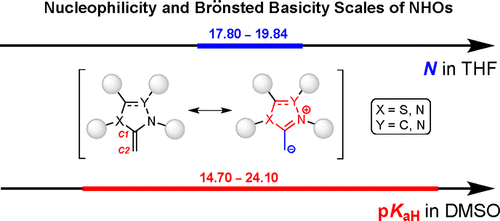
A Brönsted basicity scale comprising nine representative N-heterocyclic olefins (NHOs) was established by measuring the equilibrium acidities of their corresponding precursors in DMSO using an ultraviolet–visible spectroscopic method. The basicities (pKaHs) of the investigated NHOs cover a range from 14.7 to 24.1. The basicities of unsaturated NHOs are stronger than those of their N-heterocyclic carbene (NHC) analogues; however, the basicities for the saturated ones are much weaker than those of their NHC analogues, which is largely due to the aromatization effect that intrinsically influences the acidic dissociations of NHC and NHO precursors. The nucleophilicities of four NHOs were measured photometrically by monitoring the kinetics of reactions of these NHOs with common reference electrophiles for quantifying nucleophilic reactivities. In general, the nucleophilicity of the NHOs is much stronger than that of commonly used Lewis bases such as Ph3P or DMAP [4-(dimethylamino)pyridine] but weaker than that of their NHC analogues; however, caution should be taken when generalizing this conclusion to a wide range of electrophiles with distinctively electronic and structural properties.
中文翻译:

溶液中N-杂环烯烃的Brönsted碱和亲核性:N-杂环碳烯与N-杂环的烯烃。哪个更基本,哪个更亲核?
通过使用紫外可见光谱法测量相应的前体在DMSO中的平衡酸度,建立了包含9种代表性N-杂环烯烃(NHO)的布朗斯台德碱度标度。基本性(p K aH被调查的NHO的s)范围从14.7到24.1。不饱和NHO的碱性比其N-杂环卡宾(NHC)类似物的碱性强。但是,饱和盐的碱度比其NHC类似物的碱度弱得多,这在很大程度上是由于芳香化作用本质上影响NHC和NHO前体的酸离解。通过监测这些NHO与常用参比亲电试剂的反应动力学来光度法测量四个NHO的亲核性,以定量亲核反应性。通常,NHO的亲核性比常用的路易斯碱(例如Ph 3)强得多P或DMAP [4-(二甲氨基)吡啶],但比其NHC类似物弱;然而,将这一结论推广到具有明显电子和结构特性的各种亲电子试剂时,应谨慎行事。
更新日期:2021-02-05
中文翻译:

溶液中N-杂环烯烃的Brönsted碱和亲核性:N-杂环碳烯与N-杂环的烯烃。哪个更基本,哪个更亲核?
通过使用紫外可见光谱法测量相应的前体在DMSO中的平衡酸度,建立了包含9种代表性N-杂环烯烃(NHO)的布朗斯台德碱度标度。基本性(p K aH被调查的NHO的s)范围从14.7到24.1。不饱和NHO的碱性比其N-杂环卡宾(NHC)类似物的碱性强。但是,饱和盐的碱度比其NHC类似物的碱度弱得多,这在很大程度上是由于芳香化作用本质上影响NHC和NHO前体的酸离解。通过监测这些NHO与常用参比亲电试剂的反应动力学来光度法测量四个NHO的亲核性,以定量亲核反应性。通常,NHO的亲核性比常用的路易斯碱(例如Ph 3)强得多P或DMAP [4-(二甲氨基)吡啶],但比其NHC类似物弱;然而,将这一结论推广到具有明显电子和结构特性的各种亲电子试剂时,应谨慎行事。











































 京公网安备 11010802027423号
京公网安备 11010802027423号