当前位置:
X-MOL 学术
›
Bull. Korean Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Effect of Functional Groups on the I2 Sorption Kinetics of Isostructural Metal–Organic Frameworks
Bulletin of the Korean Chemical Society ( IF 1.7 ) Pub Date : 2021-01-19 , DOI: 10.1002/bkcs.12201 Byeongchan Lee 1 , Jinhee Park 1
Bulletin of the Korean Chemical Society ( IF 1.7 ) Pub Date : 2021-01-19 , DOI: 10.1002/bkcs.12201 Byeongchan Lee 1 , Jinhee Park 1
Affiliation
|
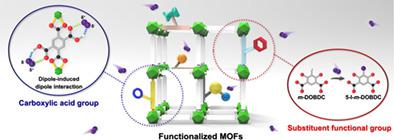
In this work, the effect of functional groups on I2 sorption kinetics is investigated using two different types of isostructural metal‐organic frameworks, UiO‐66‐X series (X = H, Br, NO2, NH2, (OH)2, and (COOH)2) and M2(m‐DOBDC) series, (M = Co2+, Mg2+, and Ni2+; m‐DOBDC4− = 4,6‐dioxo‐1,3‐benzenedicarboxylate). Among the UiO‐66‐X series, UiO‐66‐(COOH)2 exhibits the fastest sorption kinetics and the highest sorption capacity due to dipole‐induced dipole interactions between carboxylic acid groups and I2 molecules. In addition, faster I2 chemisorption is observed in M2(m‐DOBDC) because of electrophilic aromatic substitution of m‐DOBDC4− with I2. The I2 sorption mechanisms are further supported by fitting the I2 adsorption kinetics data to pseudo‐first‐order and pseudo‐second‐order kinetic models.
中文翻译:

官能团对同构金属-有机骨架的I 2吸附动力学的影响
在这项工作中,使用两种不同类型的同构金属-有机骨架UiO-66-X系列(X = H,Br,NO 2,NH 2,(OH)2)研究了官能团对I 2吸附动力学的影响,((COOH)2)和M 2(m‐ DOBDC)系列(M = Co2 +,Mg2 +和Ni2 +; m ‐ DOBDC 4- = 4,6-二氧代-1,3-苯二甲酸)。在UiO-66-X系列中,UiO-66-(COOH)2表现出最快的吸附动力学和最高的吸附能力,这归因于羧酸基团与I 2分子之间的偶极相互作用。另外,我更快2在M 2(m- DOBDC)中观察到化学吸附,因为m- DOBDC 4-被I 2亲电取代。所述I 2吸附机构由拟合我进一步支持2吸附动力学数据到伪一阶和伪二阶动力学模型。
更新日期:2021-02-24
中文翻译:

官能团对同构金属-有机骨架的I 2吸附动力学的影响
在这项工作中,使用两种不同类型的同构金属-有机骨架UiO-66-X系列(X = H,Br,NO 2,NH 2,(OH)2)研究了官能团对I 2吸附动力学的影响,((COOH)2)和M 2(m‐ DOBDC)系列(M = Co2 +,Mg2 +和Ni2 +; m ‐ DOBDC 4- = 4,6-二氧代-1,3-苯二甲酸)。在UiO-66-X系列中,UiO-66-(COOH)2表现出最快的吸附动力学和最高的吸附能力,这归因于羧酸基团与I 2分子之间的偶极相互作用。另外,我更快2在M 2(m- DOBDC)中观察到化学吸附,因为m- DOBDC 4-被I 2亲电取代。所述I 2吸附机构由拟合我进一步支持2吸附动力学数据到伪一阶和伪二阶动力学模型。



























 京公网安备 11010802027423号
京公网安备 11010802027423号