Structure ( IF 4.4 ) Pub Date : 2021-01-19 , DOI: 10.1016/j.str.2020.12.013 Sing Mei Lim 1 , Victor E Cruz 1 , Susumu Antoku 2 , Gregg G Gundersen 2 , Thomas U Schwartz 1
|
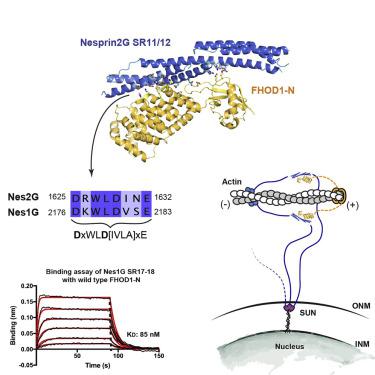
The nuclear position in eukaryotes is controlled by a nucleo-cytoskeletal network, critical in cell differentiation, division, and movement. Forces are transmitted through conserved Linker of Nucleoskeleton and Cytoskeleton (LINC) complexes that traverse the nuclear envelope and engage on either side of the membrane with diverse binding partners. Nesprin-2-giant (Nes2G), a LINC element in the outer nuclear membrane, connects to the actin directly as well as through FHOD1, a formin primarily involved in actin bundling. Here, we report the crystal structure of Nes2G bound to FHOD1 and show that the presumed G-binding domain of FHOD1 is rather a spectrin repeat (SR) binding enhancer for the neighboring FH3 domain. The structure reveals that SR binding by FHOD1 is likely not regulated by the diaphanous-autoregulatory domain helix of FHOD1. Finally, we establish that Nes1G also has one FHOD1 binding SR, indicating that these abundant, giant Nesprins have overlapping functions in actin-bundle recruitment for nuclear movement.
中文翻译:

FHOD1-Nesprin1/2 复合物的结构揭示了福尔马明 FH3 结构域的替代结合模式
真核生物中的核位置由核细胞骨架网络控制,对细胞分化、分裂和运动至关重要。力通过核骨架和细胞骨架 (LINC) 复合物的保守连接子传递,该复合物穿过核膜并在膜的任一侧与不同的结合伙伴接合。Nesprin-2-giant (Nes2G) 是外核膜中的一种 LINC 元件,它直接连接到肌动蛋白,也通过 FHOD1 连接到肌动蛋白,FHOD1 是一种主要参与肌动蛋白捆绑的形式。在这里,我们报告了与 FHOD1 结合的 Nes2G 的晶体结构,并表明 FHOD1 的假定 G 结合域是邻近 FH3 域的幽灵重复 (SR) 结合增强子。该结构表明,FHOD1 与 SR 的结合可能不受 FHOD1 的透明自动调节结构域螺旋的调节。最后,











































 京公网安备 11010802027423号
京公网安备 11010802027423号