Redox Biology ( IF 10.7 ) Pub Date : 2021-01-19 , DOI: 10.1016/j.redox.2021.101863 Xiao Cheng 1 , Mohamed Sham Shihabudeen Haider Ali 1 , Matthew Moran 1 , Martonio Ponte Viana 1 , Sarah L Schlichte 2 , Matthew C Zimmerman 3 , Oleh Khalimonchuk 4 , Mark W Feinberg 5 , Xinghui Sun 6
|
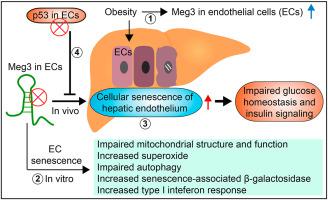
Obesity-induced insulin resistance is a risk factor for diabetes and cardiovascular disease. However, the mechanisms underlying endothelial senescence in obesity, and how it impacts obesity-induced insulin resistance remain incompletely understood. In this study, transcriptome analysis revealed that the long non-coding RNA (lncRNA) Maternally expressed gene 3 (Meg3) is one of the top differentially expressed lncRNAs in the vascular endothelium in diet-induced obese mice. Meg3 knockdown induces cellular senescence of endothelial cells characterized by increased senescence-associated β–galactosidase activity, increased levels of endogenous superoxide, impaired mitochondrial structure and function, and impaired autophagy. Moreover, Meg3 knockdown causes cellular senescence of hepatic endothelium in diet-induced obese mice. Furthermore, Meg3 expression is elevated in human nonalcoholic fatty livers and nonalcoholic steatohepatitis livers, which positively correlates with the expression of CDKN2A encoding p16, an important hallmark of cellular senescence. Meg3 knockdown potentiates obesity-induced insulin resistance and impairs glucose homeostasis. Insulin signaling is reduced by Meg3 knockdown in the liver and, to a lesser extent, in the skeletal muscle, but not in the visceral fat of obese mice. We found that the attenuation of cellular senescence of hepatic endothelium by ablating p53 expression in vascular endothelium can restore impaired glucose homeostasis and insulin signaling in obesity. In conclusion, our data demonstrate that cellular senescence of hepatic endothelium promotes obesity-induced insulin resistance, which is tightly regulated by the expression of Meg3. Our results suggest that manipulation of Meg3 expression may represent a novel approach to managing obesity-associated hepatic endothelial senescence and insulin resistance.
中文翻译:

长链非编码 RNA Meg3 缺乏通过诱导肥胖患者肝内皮细胞衰老而损害葡萄糖稳态和胰岛素信号传导
肥胖引起的胰岛素抵抗是糖尿病和心血管疾病的危险因素。然而,肥胖中内皮衰老的机制,以及它如何影响肥胖引起的胰岛素抵抗仍然不完全清楚。在本研究中,转录组分析表明,长链非编码 RNA (lncRNA) 母体表达基因 3 (Meg3) 是饮食诱导的肥胖小鼠血管内皮中差异表达最高的 lncRNA 之一。Meg3 敲低诱导内皮细胞的细胞衰老,其特征是衰老相关的 β-半乳糖苷酶活性增加、内源性超氧化物水平增加、线粒体结构和功能受损以及自噬受损。此外,Meg3 敲低导致饮食诱导的肥胖小鼠肝内皮细胞衰老。此外,CDKN2A编码 p16,这是细胞衰老的重要标志。Meg3 敲低会增强肥胖诱导的胰岛素抵抗并损害葡萄糖稳态。肝脏中的 Meg3 敲低降低了胰岛素信号传导,在较小程度上,在骨骼肌中,但在肥胖小鼠的内脏脂肪中没有。我们发现,通过消除血管内皮中 p53 的表达来减弱肝内皮细胞衰老可以恢复肥胖中受损的葡萄糖稳态和胰岛素信号传导。总之,我们的数据表明,肝内皮细胞衰老促进肥胖诱导的胰岛素抵抗,这受到 Meg3 表达的严格调节。











































 京公网安备 11010802027423号
京公网安备 11010802027423号