Molecular Catalysis ( IF 3.9 ) Pub Date : 2021-01-18 , DOI: 10.1016/j.mcat.2021.111405 Yamei Lin , Qingxia Bu , Jiaxian Xu , Xiao Liu , Xueping Zhang , Guo-Ping Lu , Baojing Zhou
|
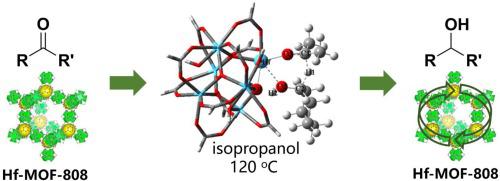
Hf-MOF-808 exhibits excellent activity and specific selectivity on the hydrogenation of carbonyl compounds via a hydrogen transfer strategy. Its superior activity than other Hf-MOFs is attributed to its poor crystallinity, defects and large specific surface area, thereby containing more Lewis acid-base sites which promote this reaction. Density functional theory (DFT) computations are performed to explore the catalytic mechanism. The results indicate that alcohol and ketone fill the defects of Hf-MOF to form a six-membered ring transition state (TS) complex, in which Hf as the center of Lewis stearic acid coordinates with the oxygen of the substrate molecule, thus effectively promoting hydrogen transfer process. Other reactive groups, such as –NO2, C = C, -CN, of inadequate hardness or large steric hindrance are difficult to coordinate with Hf, thus weakening their catalytic effect, which explains the specific selectivity Hf-MOF-808 for reducing the carbonyl group.
中文翻译:

Hf-MOF催化Meerwein-Ponndorf-Verley(MPV)还原反应:深入了解反应机理
Hf-MOF-808通过氢转移策略对羰基化合物的氢化显示出优异的活性和比选择性。其比其他Hf-MOF优异的活性归因于其较差的结晶度,缺陷和较大的比表面积,因此含有更多的路易斯酸碱位点,可促进该反应。进行密度泛函理论(DFT)计算以探索催化机理。结果表明,醇和酮可弥补Hf-MOF的缺陷,形成六元环过渡态(TS)配合物,其中以Hf为路易斯硬脂酸中心的底物与底物分子的氧配位,从而有效促进氢转移过程。其他反应性基团,例如–NO 2硬度不足或位阻大的C = C,-CN很难与Hf配位,因此减弱了它们的催化作用,这解释了Hf-MOF-808还原羰基的选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号