Journal of Organometallic Chemistry ( IF 2.1 ) Pub Date : 2021-01-18 , DOI: 10.1016/j.jorganchem.2021.121709 Li Yang , Vladimir N Nesterov , Michael G Richmond
|
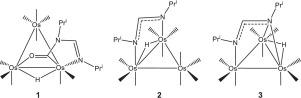
The dinitrogen donor N,N’-diisopropylformamidine [PriN=C(H)NHPri] reacts with the triosmium cluster Os3(CO)10(NCMe)2 at room temperature to yield the isomeric clusters HOs3(CO)9[μ-C(O)NPriC(H)NPri] (1) and HOs3(CO)10[μ-NPriC(H)NPri] (2) in a 1:2.8 ratio. 1 contains an edge-bridging iminocarbamoyl ligand, while 2 contains a bridging formamidinate ligand. Thermolysis of 1 yields 2 plus the face-capped cluster HOs3(CO)9[μ3-NPriC(H)NPri] (3). The decarbonylation of 2 to 3 + CO confirms the molecularity of the observed reaction steps. The three products have been fully characterized in solution by IR and NMR spectroscopies, and the solid-state structures for 1-3 have been determined by X-ray crystallography. The kinetics for the thermolysis reaction were investigated over the temperature range 342–383 K, and the concentration versus time profiles for the conversion of 1 → 2 → 3 + CO have been successfully modeled using two consecutive, irreversible first-order reactions. The bonding in clusters 1-3 have been examined by DFT, and these data support cluster 1 as the kinetic substitution product and cluster 2 as the thermodynamically favored isomer.
中文翻译:

N,N'-二异丙基甲am与[Os 3(CO)10(NCMe)2 ]反应中的双峰取代行为:甲酰胺基取代簇HOs 3(CO)9 [μ-C(O)的动力学和分子结构NPR我C(H)NPR我]的HO 3(CO)10 [μ-NPR我C(H)NPR我]和的HO 3(CO)9 [μ 3 -nPr我C(H)NPR我]
二氮供体N,N'-二异丙基甲am [Pr i N = C(H)NHPr i ]在室温下与tri簇Os 3(CO)10(NCMe)2反应,得到异构簇HOs 3(CO)9 [μ-C(O)NPR我C(H)NPR我](1)和的HO 3(CO)10 [μ-NPR我C(H)NPR我](2)以1:2.8的比例。1包含边缘桥接的亚氨基甲酰氨基配体,而2包含桥接的甲酰胺基配体。热解1产率2加上面封端的簇的HO 3(CO)9 [μ 3 -nPr我C(H)NPR我](3)。2至3 + CO的脱羰反应证实了所观察到的反应步骤的分子性。三种产品已充分表征在通过IR和NMR光谱,和固态结构的解决方案1 - 3已经通过X射线晶体学确定。在342–383 K的温度范围内研究了热解反应的动力学,以及浓度与时间的关系曲线,用于1 → 2的转化 → 已使用两个连续的,不可逆的一阶反应成功模拟了3 + CO。在群集的结合1 - 3已经由DFT检查,并且这些数据支持簇1作为动能取代产物和簇2作为热力学有利的异构体。











































 京公网安备 11010802027423号
京公网安备 11010802027423号