Journal of Cleaner Production ( IF 9.7 ) Pub Date : 2021-01-19 , DOI: 10.1016/j.jclepro.2021.126039 Xuewei Li , Xu Zhao , Xiaowen Zhou , Bo Yang
|
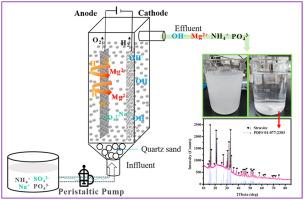
Struvite crystallization was considered to be one of the most promising ways for phosphate recovery. However, the cost of struvite recovery process was too high to be economically attractive. In this work, electrochemical-decomposition of nature magnesite was designed to economically supply magnesium and base for synthesizing struvite, and recover phosphate from aqueous solution. The continuous release of magnesium ions from solid magnesite was achieved by electrochemical action, accompanying by the generation of alkaline solution (pH: 9.0–10.8). The results showed that the electrochemical-decomposition strategy was effective to recover phosphate from aqueous solution as struvite. The key factors of phosphate recovery including current, feed rate, the molar ratio of N/P, initial concentration of phosphate and ammonium, influent pH and plate distance. For a 21-day continuous operation, the recovery rate of phosphate was between 86.6% and 93.3%, confirming the stability of the process. The economic evaluation showed that the proposed electrochemical-decomposition process can reduce the cost of phosphate recovery by up to 60% compare with the traditional chemical process, which using MgSO4·7H2O and NaOH. The struvite with typical orthorhombic crystals was obtained from the bottom of the glass container where the effluent was collected, indicating that part of the ammonium in the solution was also recovered. It was considered that the proposed process may be beneficial to increase the profitability of magnesite mining companies and wastewater treatment plants through selling-buying magnesite material, and subsequent use as a cheaper magnesium source for struvite precipitation.
中文翻译:

基于天然菱镁矿电化学分解的鸟粪石结晶从水溶液中回收磷酸盐
鸟粪石结晶被认为是回收磷酸盐最有希望的方法之一。然而,鸟粪石回收过程的成本太高而在经济上没有吸引力。在这项工作中,设计了天然菱镁矿的电化学分解方法,以经济地提供镁和碱用于合成鸟粪石,并从水溶液中回收磷酸盐。固体菱镁矿中镁离子的连续释放是通过电化学作用实现的,同时产生碱性溶液(pH:9.0-10.8)。结果表明,电化学分解策略可有效地从水溶液中回收鸟粪石中的磷酸盐。磷酸盐回收的关键因素包括电流,进料速度,N / P摩尔比,磷酸盐和铵的初始浓度,进水pH值和塔板距离。对于21天的连续操作,磷酸盐的回收率在86.6%和93.3%之间,证实了该工艺的稳定性。经济评估表明,与传统的使用MgSO4的化学工艺相比,本发明的电化学分解工艺可将磷酸盐回收的成本降低多达60%。4 ·7H 2 O和NaOH。从玻璃容器的底部收集了流出物,得到了具有典型斜方晶体的鸟粪石,表明溶液中的一部分铵也被回收了。有人认为,拟议的工艺可能有利于通过买卖菱镁矿材料并随后用作廉价的镁锰矿沉淀物来提高菱镁矿开采公司和废水处理厂的盈利能力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号