Colloids and Surfaces A: Physicochemical and Engineering Aspects ( IF 4.9 ) Pub Date : 2021-01-19 , DOI: 10.1016/j.colsurfa.2021.126169 Yan Zhang , Qian Li , Shaokang Sun , Xiaoliang Liu , Tao Jiang , Xianjun Lyu , Yinghe He
|
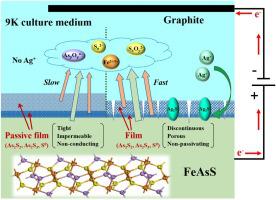
Arsenopyrite (FeAsS) is the most common As-containing sulphide mineral in gold deposits. Most auriferous arsenopyrite ores are refractory necessitating pre-treatments before gold extraction. Bio-oxidation pre-treatment is simple and eco-friendly technology that has attracted significant attention in the past decade. Slow leaching kinetics of bioleaching is, however, a critical impedance to its large-scale application. The addition of metal cations has previously been found to expedite the leaching process. The electrochemical behaviour of Ag+ in the dissolution of arsenopyrite in 9 K culture medium was investigated in depth by a series of electrochemical and analytical techniques. Electrochemical results suggested that Ag+ could significantly improve the oxidative dissolution of arsenopyrite. Analytical results demonstrated that, with Ag+, only a discontinuous, porous and non-passivating film was formed on the arsenopyrite surface. The catalytic effect of silver is largely attributed to that Ag+ can enhance the oxidative dissolution of not only arsenopyrite but also the passive film by forming conducting Ag2S on the arsenopyrite surface, which explains why Ag+ can remove the passive film on the surface of arsenopyrite during bioleaching and thus effectively shorten its bioleaching period.
中文翻译:

Ag +在9K培养基中催化毒砂氧化溶解的电化学行为。
毒砂(FeAsS)是金矿中最常见的含砷硫化物矿物。大多数金铁毒砂矿石都是难处理的,因此必须在提取金之前进行预处理。生物氧化预处理是一种简单且环保的技术,在过去的十年中引起了极大的关注。然而,生物浸出的缓慢浸出动力学是其大规模应用的关键阻抗。先前已经发现添加金属阳离子可以加速浸出过程。通过一系列电化学和分析技术,深入研究了Ag +在毒砂在9 K培养基中溶解的电化学行为。电化学结果表明,Ag +可以显着改善毒砂的氧化溶解。分析结果表明,使用Ag +时,毒砂表面仅形成不连续的多孔非钝化膜。银的催化作用主要归因于Ag +可以通过在毒砂表面上形成导电Ag 2 S来增强毒砂和被动膜的氧化溶解,这解释了为什么Ag +可以去除表面上的被动膜。生物浸出过程中毒砂的残留,从而有效地缩短了其生物浸出时间。











































 京公网安备 11010802027423号
京公网安备 11010802027423号