Archives of Biochemistry and Biophysics ( IF 3.8 ) Pub Date : 2021-01-19 , DOI: 10.1016/j.abb.2021.108767 José L. Neira , Sonia Vega , Sergio Martínez-Rodríguez , Adrián Velázquez-Campoy
|
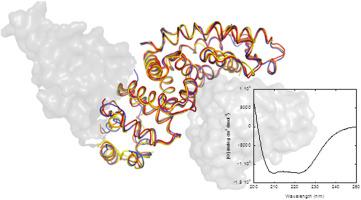
Neurofibromin-1 (NF1) is a large, multidomain tumour suppressor encoded by the NF1 gene. The gene is mutated in neurofibromatosis type I, a disease characterized by malignant tumours of the nervous system and benign neurofibromas. The best-known activity of NF1 is the down-regulation of the mitogen-activated protein kinase pathway via its three-hundred-residue-long GTPase-activating protein (GAP) domain (the so-called GAP-related domain (NF1-GRD)). The NF1-GRD stimulates Ras GTPase activity in turning off signalling. Despite this activity, NF1-GRD has been demonstrated to bind to other different proteins, such as SPRED1 or MC1R. We have embarked on the biophysical and conformational characterization of NF1-GRD in solution by using several spectroscopic (namely fluorescence and circular dichroism (CD)) and biophysical techniques (namely size exclusion chromatography (SEC) and differential scanning calorimetry (DSC)). This biophysical characterization is crucial in deciphering NF1-GRD interactome and in finding biochemical features, modulating possible protein interactions. The native-like structure of NF1-GRD (as monitored by intrinsic fluorescence and far-UV CD) was strongly pH-dependent showing a pH-titration causing a substantial increase in its helicity. NF1-GRD had a low conformational stability, as concluded from DSC experiments and thermal denaturations followed by intrinsic and ANS fluorescence, and CD. Chemical denaturations showed that NF1-GRD unfolded through an intermediate which has a substantial amount of solvent-exposed hydrophobic patches.
中文翻译:

分离的神经纤维蛋白1的GTPase激活蛋白相关结构域在溶液中构象稳定性低
Neurofibromin-1(NF1)是由NF1基因编码的大型多域肿瘤抑制因子。该基因在I型神经纤维瘤病中发生突变,该疾病以神经系统恶性肿瘤和良性神经纤维瘤为特征。NF1最著名的活性是通过以下途径下调有丝分裂原激活的蛋白激酶途径它的三百个残基长的GTPase激活蛋白(GAP)域(所谓的GAP相关域(NF1-GRD))。NF1-GRD通过关闭信号传导刺激Ras GTPase活性。尽管具有这种活性,但已证明NF1-GRD可以结合其他不同的蛋白质,例如SPRED1或MC1R。我们已经通过使用几种光谱学(即荧光和圆二色性(CD))和生物物理技术(即尺寸排阻色谱法(SEC)和差示扫描量热法(DSC)),对溶液中NF1-GRD的生物物理和构象进行了表征。这种生物物理特征对于破译NF1-GRD相互作用组和发现生化特征,调节可能的蛋白质相互作用至关重要。NF1-GRD的天然结构(通过固有荧光和远紫外线CD监测)强烈地依赖于pH值,显示pH滴定导致其螺旋度显着增加。NF1-GRD具有低构象稳定性,这是通过DSC实验和热变性以及固有荧光和ANS荧光以及CD得出的结论。化学变性显示NF1-GRD通过具有大量溶剂暴露的疏水性斑块的中间体展开。











































 京公网安备 11010802027423号
京公网安备 11010802027423号