当前位置:
X-MOL 学术
›
Immunology
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The immunopeptidomes of two transmissible cancers and their host have a common, dominant peptide motif
Immunology ( IF 4.9 ) Pub Date : 2021-01-18 , DOI: 10.1111/imm.13307 Annalisa Gastaldello 1 , Sri H Ramarathinam 2 , Alistair Bailey 3, 4 , Rachel Owen 1 , Steven Turner 3 , N Kontouli 3 , Tim Elliott 3, 4 , Paul Skipp 1, 4 , Anthony W Purcell 2 , Hannah V Siddle 1, 4
Immunology ( IF 4.9 ) Pub Date : 2021-01-18 , DOI: 10.1111/imm.13307 Annalisa Gastaldello 1 , Sri H Ramarathinam 2 , Alistair Bailey 3, 4 , Rachel Owen 1 , Steven Turner 3 , N Kontouli 3 , Tim Elliott 3, 4 , Paul Skipp 1, 4 , Anthony W Purcell 2 , Hannah V Siddle 1, 4
Affiliation
|
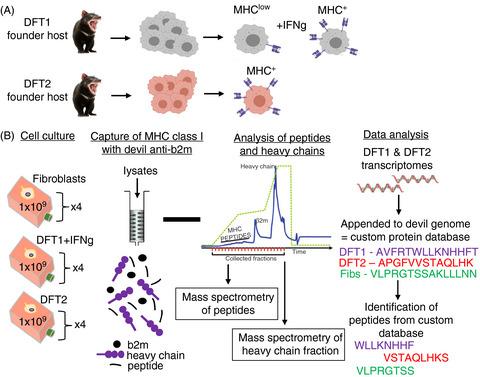
Transmissible cancers are malignant cells that can spread between individuals of a population, akin to both a parasite and a mobile graft. The survival of the Tasmanian devil, the largest remaining marsupial carnivore, is threatened by the remarkable emergence of two independent lineages of transmissible cancer, devil facial tumour (DFT) 1 and devil facial tumour 2 (DFT2). To aid the development of a vaccine and to interrogate how histocompatibility barriers can be overcome, we analysed the peptides bound to major histocompatibility complex class I (MHC‐I) molecules from Tasmanian devil cells and representative cell lines of each transmissible cancer. Here, we show that DFT1 + IFN‐γ and DFT2 cell lines express a restricted repertoire of MHC‐I allotypes compared with fibroblast cells, potentially reducing the breadth of peptide presentation. Comparison of the peptidomes from DFT1 + IFNγ, DFT2 and host fibroblast cells demonstrates a dominant motif, despite differences in MHC‐I allotypes between the cell lines, with preference for a hydrophobic leucine residue at position 3 and position Ω of peptides. DFT1 and DFT2 both present peptides derived from neural proteins, which reflects a shared cellular origin that could be exploited for vaccine design. These results suggest that polymorphisms in MHC‐I molecules between tumours and host can be ‘hidden’ by a common peptide motif, providing the potential for permissive passage of infectious cells and demonstrating complexity in mammalian histocompatibility barriers.
中文翻译:

两种传染性癌症及其宿主的免疫肽组具有共同的显性肽基序
传染性癌症是一种恶性细胞,可以在群体中的个体之间传播,类似于寄生虫和移动移植物。袋獾是现存最大的有袋动物食肉动物,它的生存受到两个独立的传染性癌症谱系——魔鬼面部肿瘤 (DFT) 1 和魔鬼面部肿瘤 2 (DFT2) 的显着出现的威胁。为了帮助开发疫苗并询问如何克服组织相容性障碍,我们分析了与来自塔斯马尼亚恶魔细胞和每种传染性癌症的代表性细胞系的主要组织相容性复合体 I 类 (MHC-I) 分子结合的肽。在这里,我们表明与成纤维细胞相比,DFT1 + IFN-γ 和 DFT2 细胞系表达有限的 MHC-I 同种异型库,可能会降低肽呈递的广度。来自 DFT1 + IFNγ、DFT2 和宿主成纤维细胞的肽组的比较表明,尽管细胞系之间的 MHC-I 同种异型存在差异,但优先选择肽的 3 位和 Ω 位的疏水性亮氨酸残基。DFT1 和 DFT2 都呈现源自神经蛋白的肽,这反映了可用于疫苗设计的共享细胞起源。这些结果表明,肿瘤和宿主之间 MHC-I 分子的多态性可以被一个共同的肽基序“隐藏”,为传染性细胞的允许通过提供了潜力,并证明了哺乳动物组织相容性障碍的复杂性。优先选择肽的位置 3 和位置 Ω 处的疏水性亮氨酸残基。DFT1 和 DFT2 都呈现源自神经蛋白的肽,这反映了可用于疫苗设计的共享细胞起源。这些结果表明,肿瘤和宿主之间 MHC-I 分子的多态性可以被一个共同的肽基序“隐藏”,为传染性细胞的允许通过提供了潜力,并证明了哺乳动物组织相容性障碍的复杂性。优先选择肽的位置 3 和位置 Ω 处的疏水性亮氨酸残基。DFT1 和 DFT2 都呈现源自神经蛋白的肽,这反映了可用于疫苗设计的共享细胞来源。这些结果表明,肿瘤和宿主之间 MHC-I 分子的多态性可以被一个共同的肽基序“隐藏”,为传染性细胞的允许通过提供了潜力,并证明了哺乳动物组织相容性障碍的复杂性。
更新日期:2021-01-18
中文翻译:

两种传染性癌症及其宿主的免疫肽组具有共同的显性肽基序
传染性癌症是一种恶性细胞,可以在群体中的个体之间传播,类似于寄生虫和移动移植物。袋獾是现存最大的有袋动物食肉动物,它的生存受到两个独立的传染性癌症谱系——魔鬼面部肿瘤 (DFT) 1 和魔鬼面部肿瘤 2 (DFT2) 的显着出现的威胁。为了帮助开发疫苗并询问如何克服组织相容性障碍,我们分析了与来自塔斯马尼亚恶魔细胞和每种传染性癌症的代表性细胞系的主要组织相容性复合体 I 类 (MHC-I) 分子结合的肽。在这里,我们表明与成纤维细胞相比,DFT1 + IFN-γ 和 DFT2 细胞系表达有限的 MHC-I 同种异型库,可能会降低肽呈递的广度。来自 DFT1 + IFNγ、DFT2 和宿主成纤维细胞的肽组的比较表明,尽管细胞系之间的 MHC-I 同种异型存在差异,但优先选择肽的 3 位和 Ω 位的疏水性亮氨酸残基。DFT1 和 DFT2 都呈现源自神经蛋白的肽,这反映了可用于疫苗设计的共享细胞起源。这些结果表明,肿瘤和宿主之间 MHC-I 分子的多态性可以被一个共同的肽基序“隐藏”,为传染性细胞的允许通过提供了潜力,并证明了哺乳动物组织相容性障碍的复杂性。优先选择肽的位置 3 和位置 Ω 处的疏水性亮氨酸残基。DFT1 和 DFT2 都呈现源自神经蛋白的肽,这反映了可用于疫苗设计的共享细胞起源。这些结果表明,肿瘤和宿主之间 MHC-I 分子的多态性可以被一个共同的肽基序“隐藏”,为传染性细胞的允许通过提供了潜力,并证明了哺乳动物组织相容性障碍的复杂性。优先选择肽的位置 3 和位置 Ω 处的疏水性亮氨酸残基。DFT1 和 DFT2 都呈现源自神经蛋白的肽,这反映了可用于疫苗设计的共享细胞来源。这些结果表明,肿瘤和宿主之间 MHC-I 分子的多态性可以被一个共同的肽基序“隐藏”,为传染性细胞的允许通过提供了潜力,并证明了哺乳动物组织相容性障碍的复杂性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号